Compound licorice preparation for eliminating phlegm and relieving cough and preparation method thereof
A technology of compound licorice and preparation, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, pill delivery, etc., to achieve the effects of reasonable formula, reduced usage, and reduced curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
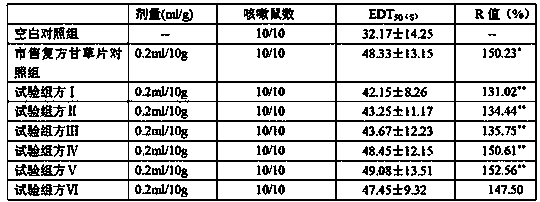
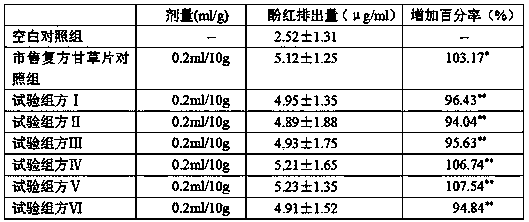
[0024] Licorice extract powder 450g, pentoxyverine citrate 3g, opium powder 14.08g, camphor 8g, star anise oil 8g, sodium benzoate 8g, sodium bicarbonate 378.13g, tartaric acid 343.76g, sodium carboxymethyl starch 57.29g, Lactose 171.88g, cross-linked polyvinylpyrrolidone 58.44g, polyethylene glycol 6000 66.46g, micropowder silica gel 45.83g, aspartame 13.32g, orange flavor 11.14g (licorice extract powder in the formula, citric acid spray Toverine, opium powder, camphor, and star anise oil are products prepared in accordance with the standards of the Chinese Pharmacopoeia 2010 edition, and are commercially available products; while sodium benzoate, sodium bicarbonate, tartaric acid, sodium carboxymethyl starch, lactose, and cross-linked polyethylene Pyrrolidone, polyethylene glycol 6000, micropowder silica gel, aspartame, and orange flavor essence are also commercially available pharmaceutical excipients. Other examples are the same as Example 1).
[0025] The preparation proc...
Embodiment 2
[0032] Licorice extract powder 450g, pentoxyverine citrate 3.35g, opium powder 13.85g, camphor 8g, star anise oil 8g, sodium benzoate 8g, sodium bicarbonate 501.02g, tartaric acid 442.08g, sodium carboxymethyl starch 76.63g , lactose 294.72g, cross-linked polyvinylpyrrolidone 79.57g, polyethylene glycol 6000 85.47g, micronized silica gel 44.26g, aspartame 15.8g, orange flavor 14.1g.
[0033] The preparation process comprises the following steps:
[0034] 1) Dissolve the above formula amount of camphor in the formula amount of star anise oil, and then add the camphor-dissolved star anise oil into the formula amount of micropowdered silica gel dried at 70°C with a water content of ≤1.5% and passed through a 80-mesh sieve. Stir and mix evenly, seal and place for 18 hours to obtain adsorbate powder.
[0035] 2) Same as Step 2 of Example 1.
[0036] 3). Same as Step 3 of Example 1.
[0037] 4) Dissolve polyethylene glycol 6000 with 75% (volume fraction) ethanol, then dissolve so...
Embodiment 3
[0040] Licorice extract powder 450g, pentoxyverine citrate 4.49g, opium powder 13.12g, camphor 8g, star anise oil 8g, sodium benzoate 8g, sodium bicarbonate 592.61g, tartaric acid 522.89g, sodium carboxymethyl starch 87.14g , lactose 278.81g, cross-linked polyvinylpyrrolidone 90.63g, polyethylene glycol 6000 78.43g, micronized silica gel 63.72g, aspartame 16.32g, orange flavor 12.66g.
[0041] The preparation process comprises the following steps:
[0042] 1) Dissolve the above formula amount of camphor in the formula amount of star anise oil, then add the camphor-dissolved star anise oil into the formula amount of micropowder silica gel dried at 70°C with a moisture content of ≤1.5% and pass through a 80 mesh sieve, and stir Mix evenly and seal for 16 hours to obtain adsorbate powder.
[0043] 2) Same as Step 2 of Example 1.
[0044] 3) Same as Step 3 of Example 1.
[0045] 4) Same as Step 4 of Example 2.
[0046] 5) Same as Step 5 of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com