Novel amido-linkage imidazolium salt gemini surfactant and preparation method thereof
A Gemini surface and amide bond technology, which is applied in chemical instruments and methods, dissolution, organic chemistry, etc., can solve the problems of high reaction temperature, long reaction time, and solvent toxicity, and achieve low reaction temperature, short reaction time, and solvent toxicity. small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
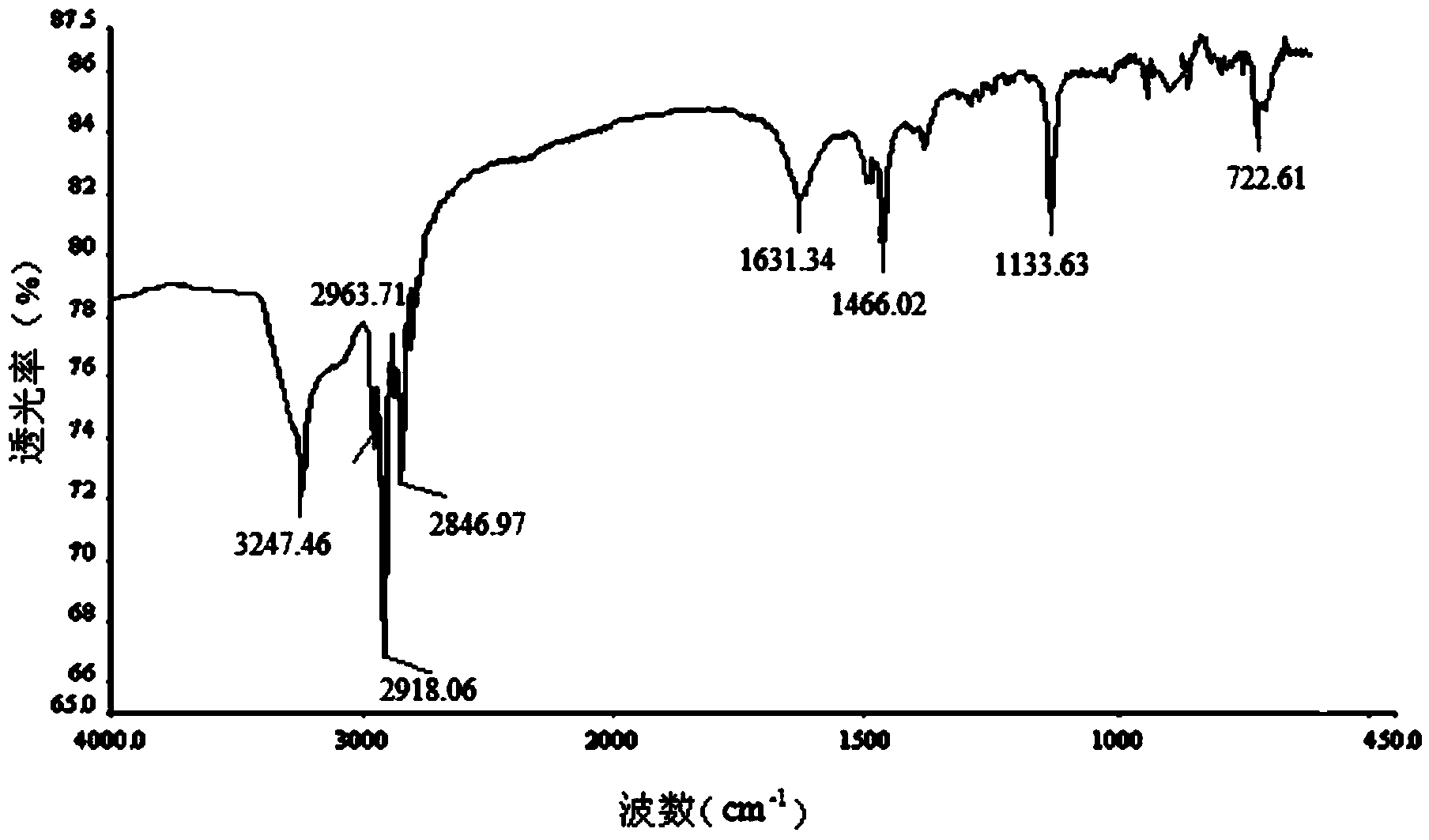
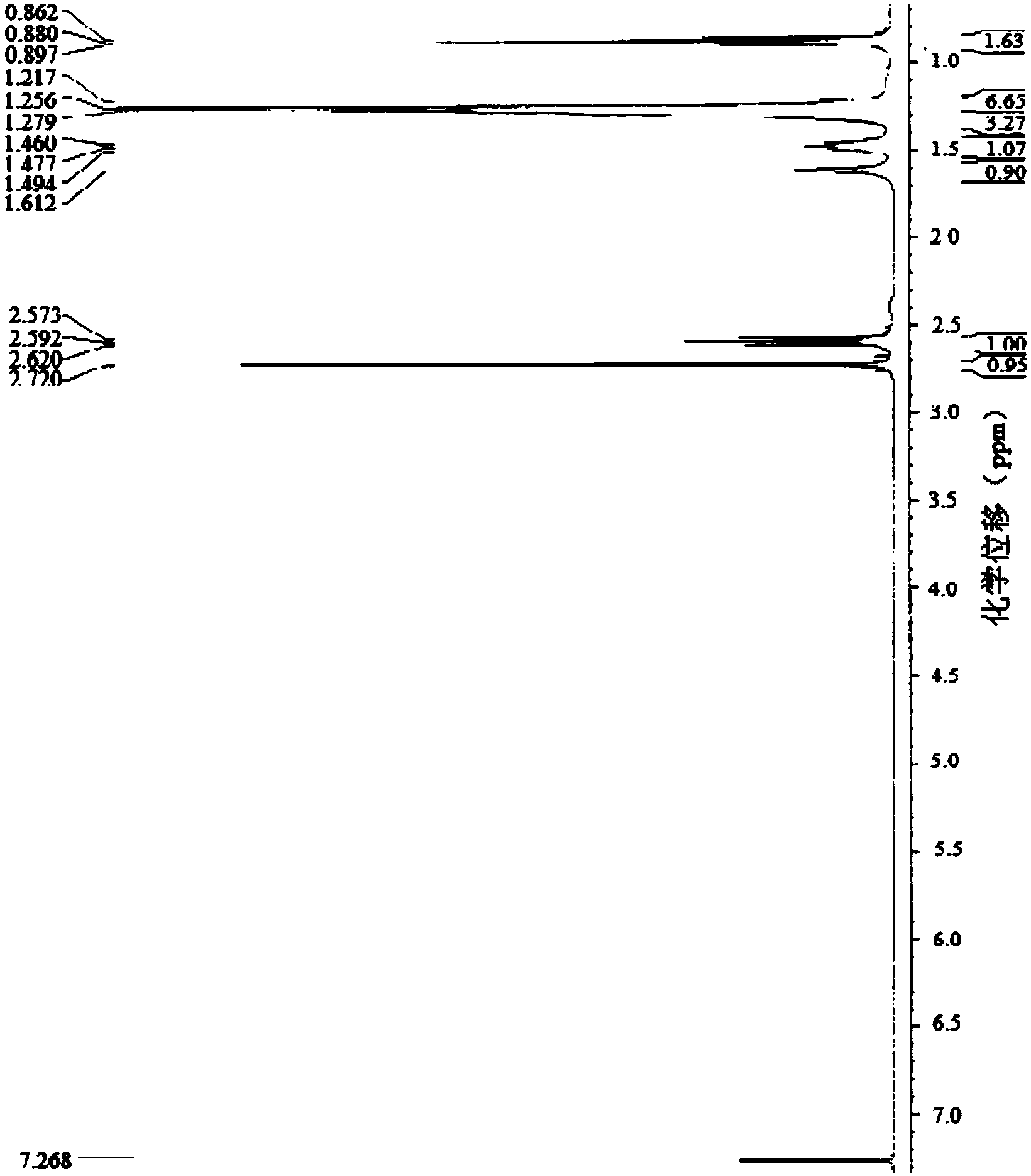
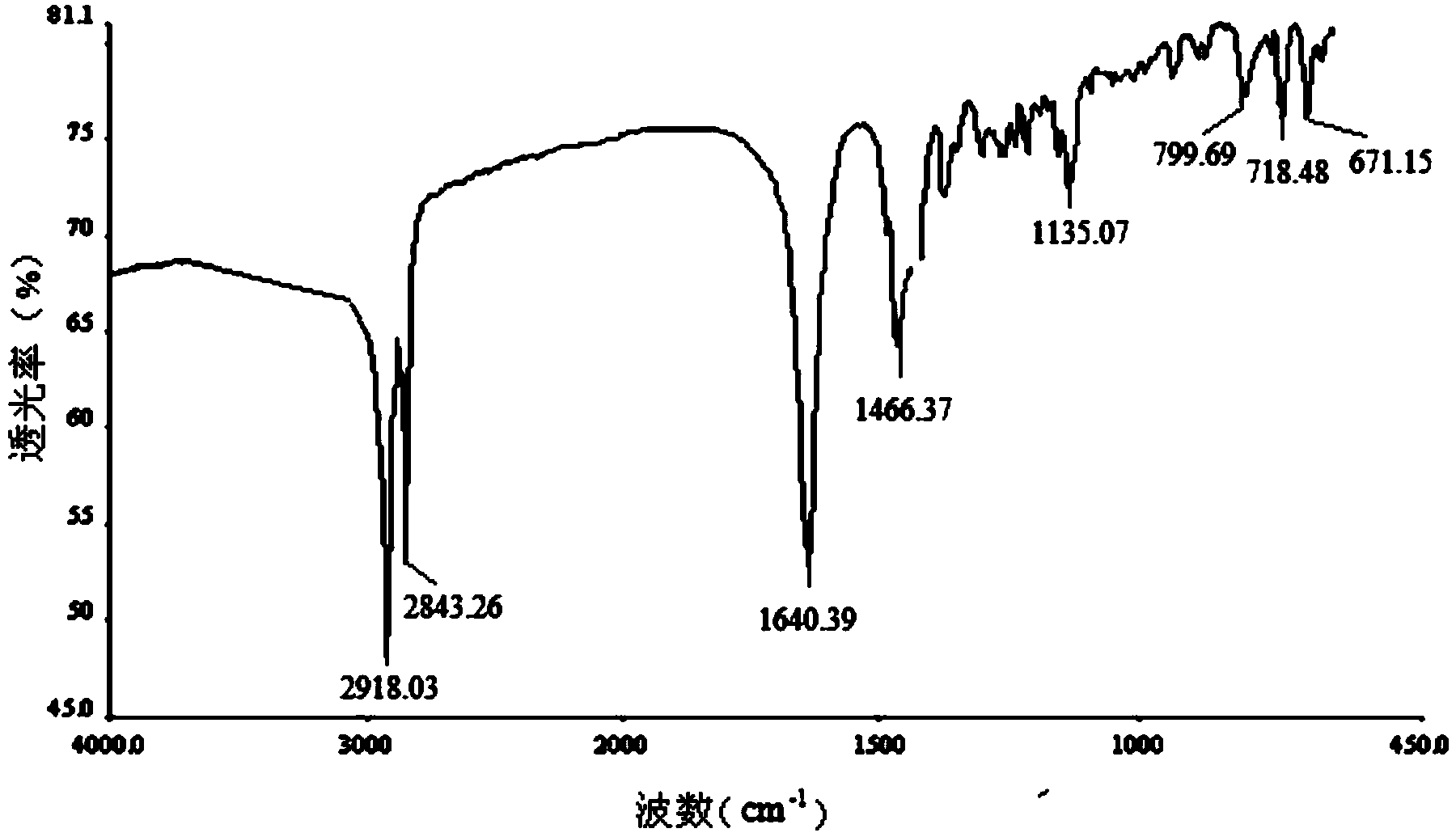
Image
Examples
specific Embodiment approach 1
[0022] Specific embodiment one: The general structural formula of the novel amide-linked imidazolium-based gemini surfactant of the present embodiment is as follows:
[0023]
[0024] where R=C n h 2n+1 , n=6, 8, 10, 12, 14, 16, 18.
specific Embodiment approach 2
[0025] Specific embodiment two: the preparation method of the novel amide linkage imidazolium base gemini surfactant of the present embodiment is carried out according to the following steps:
[0026] 1. Add ethylenediamine and 1-bromoalkane to the dimethylformamide solvent, then add NaOH, and react for 26h to 30h at room temperature and at a stirring speed of 800r / pm to 1200r / pm, and the reaction is complete After filtration, a white solid was obtained. The white solid was washed 3 to 5 times with a NaOH aqueous solution with a mass fraction of 50% to 70%, and then recrystallized with an aqueous ethanol solution. Dry at 30-35°C for 5h-6h to obtain N,N'-dialkylethylenediamine; wherein the chemical formula of the 1-bromoalkane and 1-bromoalkane is C n h 2n+1Br, n=6, 8, 10, 12, 14, 16, 18, the ratio of the amount of ethylenediamine to 1-bromoalkane is 1: (2~3), the dimethyl The ratio of the volume of methyl formamide to the amount of 1-bromoalkane is 20mL: (0.02-0.03) mol, and...
specific Embodiment approach 3
[0031] Embodiment 3: This embodiment is different from Embodiment 2 in that the ratio of the amount of ethylenediamine to 1-bromoalkane in step 1 is 1:2.75. Other steps and parameters are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com