Pridinol mesylate diclofenac sodium injection and preparation method thereof
A technology of nordiclofenac sodium and diclofenac sodium, which is applied in the field of pritinol mesylate preparation and its preparation, can solve the problems of decreased stability, failure to meet the concentration requirements of the preparation, low solubility, etc., and achieve low cost and meet the requirements of clinical treatment Need, Prescription Advanced Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1, Prescription Research of Pridino Mesylate Diclofenac Sodium Injection
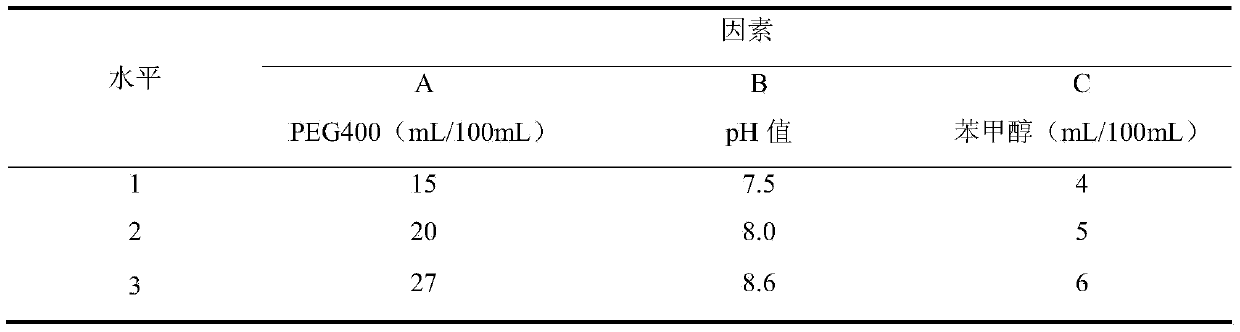
[0017] Because the dissolubility of pridinol mesylate and diclofenac sodium in water is all small, can not reach preparation concentration requirement; And, pridinol mesylate is a kind of weakly acidic salt, and diclofenac sodium is weak base in aqueous solution When the two are directly dissolved in water and then mixed, an acid-base reaction will occur to generate pridinol and diclofenac with less solubility and precipitate out of the solution. Therefore, finding a suitable solvent system is the technical key for preparing pridinol mesylate diclofenac sodium injection. After trial and error, the inventor finally determined to use water for injection and polyethylene glycol as the mixed solvent.
[0018] The structure of diclofenac sodium contains easily oxidizable groups and is unstable in aqueous solution. Therefore, improving the stability of the preparation is also the technical...
Embodiment 2
[0029] Embodiment 2, the preparation of pridinol mesylate diclofenac sodium injection
[0030] The optimal prescription screened out in Example 1 is used to prepare the pritinol mesylate diclofenac sodium injection, the preparation method is as follows: take 10mL of water for injection, add the pritinol mesylate of the prescribed amount, stir until completely dissolved, and obtain solution A Take 10 mL of water for injection, add mannitol and sodium metabisulfite in the prescribed amount, and stir until completely dissolved to obtain solution B; slowly add solution B to solution A under slow stirring conditions to obtain solution C; additionally take 10 mL of water for injection and Add the prescribed amount of PEG400, add the prescribed amount of diclofenac sodium, stir until completely dissolved, and obtain solution D; slowly add solution D to solution C under slow stirring conditions, then add the prescribed amount of benzyl alcohol, stir and mix, add Water for injection to...
Embodiment 3
[0032] Embodiment 3, the osmotic pressure determination of pridinol mesylate diclofenac sodium injection
[0033] Using the "Chinese Pharmacopoeia" 2010 edition two Appendix IX "Determination of Osmotic Pressure Molar Concentration", measure the osmotic pressure of Pridino Mesylate Diclofenac Sodium Injection. The result shows that the osmolality of the pridinol mesylate diclofenac sodium injection that embodiment 2 makes is 300.1mOsmol, and the normal human blood osmolality is 285~310mOsmol / kg, therefore, the mesylate prepared by the present invention Puridinol Acid Diclofenac Sodium Injection meets the osmotic pressure requirements.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com