Amino acid zwitter ion hydrogel material and preparation method thereof
A zwitterion and amino acid technology, applied in the field of functional polymer materials, can solve the problems of high price of zwitterion monomers, complicated preparation methods, expensive phosphorylcholine monomers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
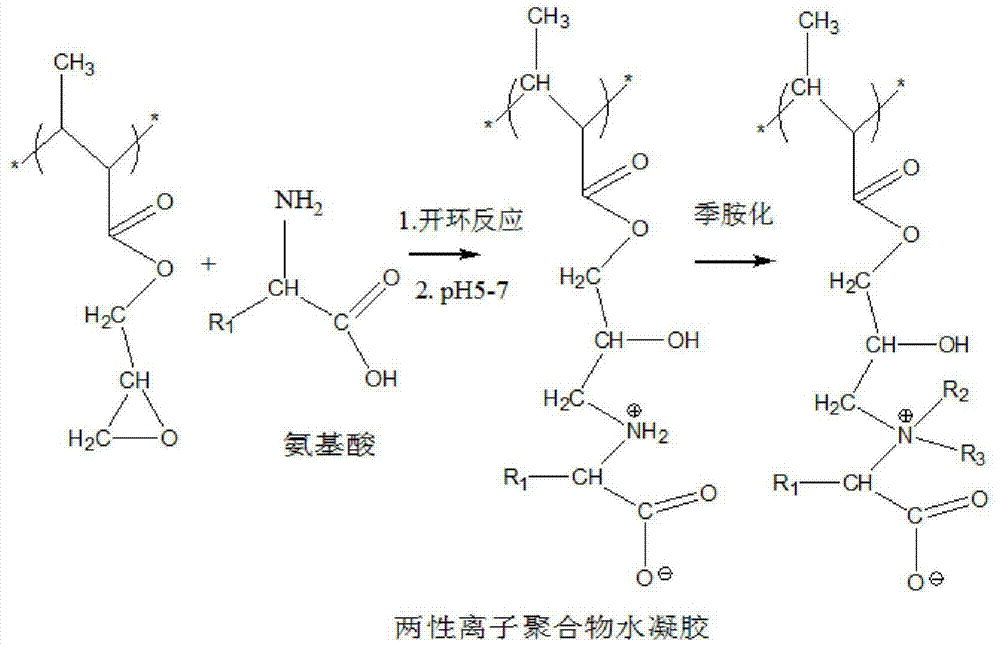
[0040] The preparation method of the amino acid zwitterionic hydrogel material is: glycidyl (meth)acrylate, at least one or more than one polymerizable monomer, at least one or more than one cross-linking agent for the monomer, at least one A polymerizable composition composed of a free radical initiator is polymerized under ultraviolet light or thermal initiation conditions to obtain a cross-linked polymer product, the polymer product is soaked in a solution containing an amino acid or a polypeptide, and further combined with a terminal primary amino group of the amino acid or polypeptide Or the secondary amino group is subjected to an epoxy ring-opening reaction under alkaline conditions of pH 8-14, and the temperature is 30°C to 150°C for 0.5 hours to 3 days to obtain a hydrogel material, and then adjust the pH to about 7, A zwitterionic hydrogel material is obtained. Or further obtain the methylated amino acid zwitterionic hydrogel material by reacting with methyl iodide. ...
example 1
[0062] 2 g of β-hydroxyethyl methacrylate, 1 g of glycidyl methacrylate, 0.015 g of ethylene glycol dimethacrylate, 0.015 g of initiator alkyl aryl ketone Darocur-1173, mix well and inject into the mold In the process, it is cured by ultraviolet light, demolded, soaked and washed, and placed in physiological saline to obtain epoxy hydrogel. The material is optically transparent, with a water content of 25%, a Young's modulus of 1.4MPa, a water contact angle of 78 degrees, and a protein adsorption capacity of 80μg / cm 2 ,RPD 10 35%.
example 2
[0064] Place 2 grams of the epoxy hydrogel in Example 1 in a glycine solution with a concentration of 10%, add sodium hydroxide solution to adjust the pH to 13, heat to 40°C, react for 24 hours, then wash with water, and soak in In a buffer solution with a pH of 7, a glycine zwitterionic hydrogel material is obtained. The material is optically transparent, with a water content of 60%, a water contact angle of 35 degrees, and a protein adsorption capacity of 19 μg / cm 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| water contact angle | aaaaa | aaaaa |
| water contact angle | aaaaa | aaaaa |
| water contact angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com