Spectrophotometry quantitative analysis method of iodine-starch color-developing system
A quantitative analysis and spectrophotometric technology, which is applied in the field of chemical and spectral analysis and testing, can solve the problems of unstable iodine-starch color reaction and low reliability of analysis results, and achieve high accuracy, easy operation and maximum reliability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
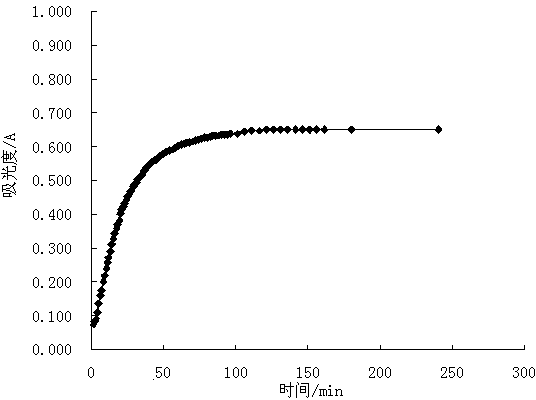
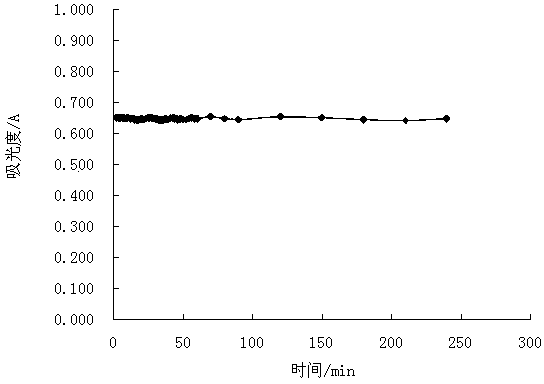
[0032] Example 1: Fe 3+ and H 2 o 2 The stability test and the establishment of the standard curve of the equilibrium system co-constructed with iodine-starch, the operation steps:
[0033] (1) Pipette 0.00, 0.20, 0.40, 0.60, 0.80, 1.00 mL of I - Standard solution (2.50 mmol / mL) in 25 mL volumetric flask (number 0~5), add 1.5 mol / L FeCl 3 Solution 50 μL, shake well, let it stand for 30 min, add 3.00 mL of 1% starch solution, shake well, add distilled water near the neck of the volumetric flask, shake well, add 1.5% H 2 o 2 50 μL, shake well, make up to volume with distilled water, shake well;
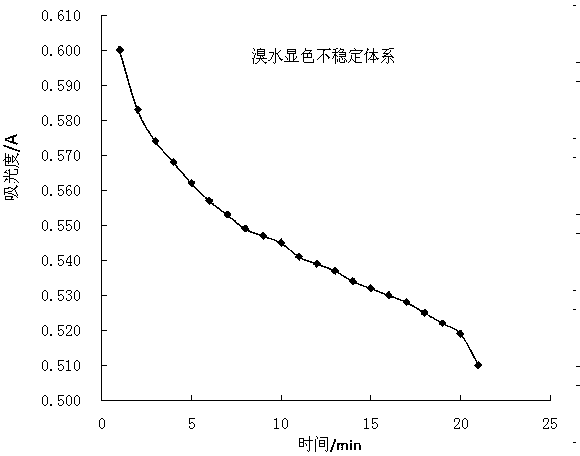
[0034] (2) At room temperature, use No. 0 bottle as blank, use No. 4 bottle as wavelength scanning at 400-700nm, and draw the absorption curve ( Figure 5 ), the maximum absorption wavelength is 600nm;
[0035] (3) At room temperature, with 600nm as the measurement wavelength, measure the absorbance of bottles No. 1 to No. 5; measure once every 30 minutes, draw a standard curve ...
Embodiment 2
[0043] Embodiment 2: with Fe 3+ and H 2 o 2 Build an equilibrium system with iodine-starch to determine the iodine content of a certain commercially available potassium iodide by spectrophotometry, the operation steps:
[0044] (1) Accurately weigh 0.1g (accurate to 0.0001g) of commercially available KI (Chengdu Kelong Reagent Factory), dissolve it with an appropriate amount of distilled water, transfer to a 100mL volumetric flask to constant volume, pipette 5.00mL of the solution into a 50mL volumetric flask, Constant volume with distilled water to obtain the test solution;
[0045] (2) Pipette 0.00, 0.20, 0.40, 0.60, 0.80, 1.00 mL of I - Standard solution (2.50 mmol / mL) in 25mL volumetric flask (number 0~5), respectively pipette 0.60mL of test solution into 25mL volumetric flask ( n =6); respectively add 1.5mol / L FeCl 3 50 μL of the solution, shake well, let stand for 30 min to fully react, add 3.00 mL of 1% starch solution, shake well, add distilled water near the neck...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com