Preparation method of palladium-lead bimetallic catalyst with eggshell structure
A bimetallic catalyst, catalyst technology, applied in catalyst activation/preparation, preparation of organic compounds, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of low catalytic efficiency, high noble metal loading, and cumbersome preparation process And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Put 5g of spherical alumina carrier into a 500mL beaker, add deionized water, put it in a water bath, add 4.0mL of H 2 PdCl 4 The solution was heated to 60°C. 20 mL of n-butanol was added dropwise, and the reaction was stopped after 0.5 h. Cool to room temperature, filter with suction, wash with plenty of water, and dry in a vacuum oven at 40°C for 12 hours. Gained catalyst is counted as: Pd 2 / Al 2 o 3 . The contents of Pd and Pb in the catalyst were measured by ICP-AES, see Table 1. Example 2
Embodiment 2
[0022] 0.16g of lead acetylacetonate was dissolved in deionized water, added to the alumina carrier, allowed to stand, and then put into a vacuum drying oven to dry for 12 hours. Calcined at 300°C for 0.5h in air atmosphere. Put the impregnated-roasted lead-containing alumina carrier into a 500mL beaker, add deionized water, put it in a water bath, add 4.6mL of palladium acetate solution under mechanical stirring, and raise the temperature to 60°C. 25mL of 37wt% formaldehyde solution was added dropwise, and the reaction was stopped after 6h. Cool to room temperature, filter with suction, wash with plenty of water, and dry in a vacuum oven at 80°C for 12 hours. Gained catalyst is counted as: Pd 1 Pb 1 / Al 2 o 3 . The contents of Pd and Pb in the catalyst were measured by ICP-AES, see Table 1.
Embodiment 3
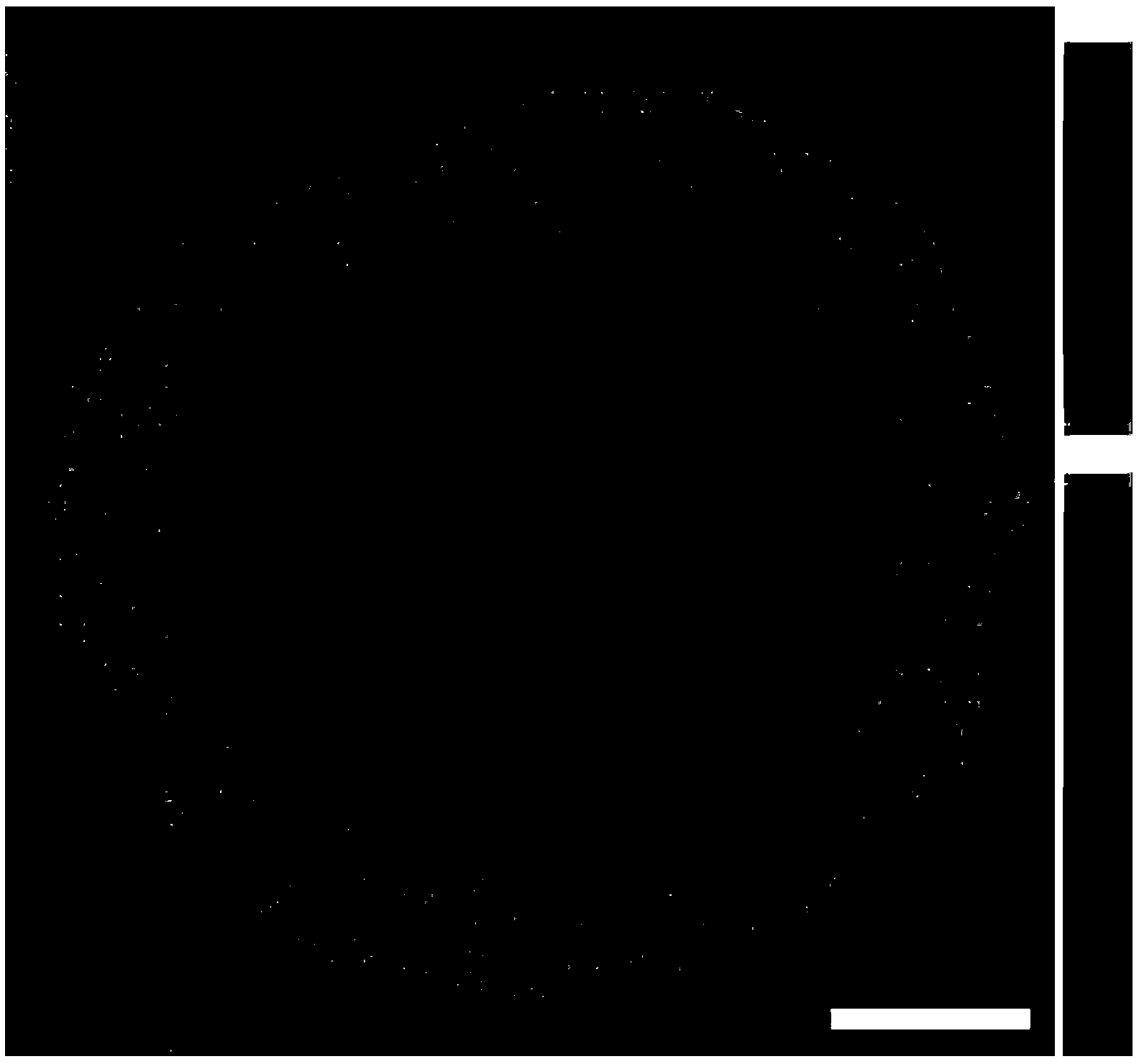
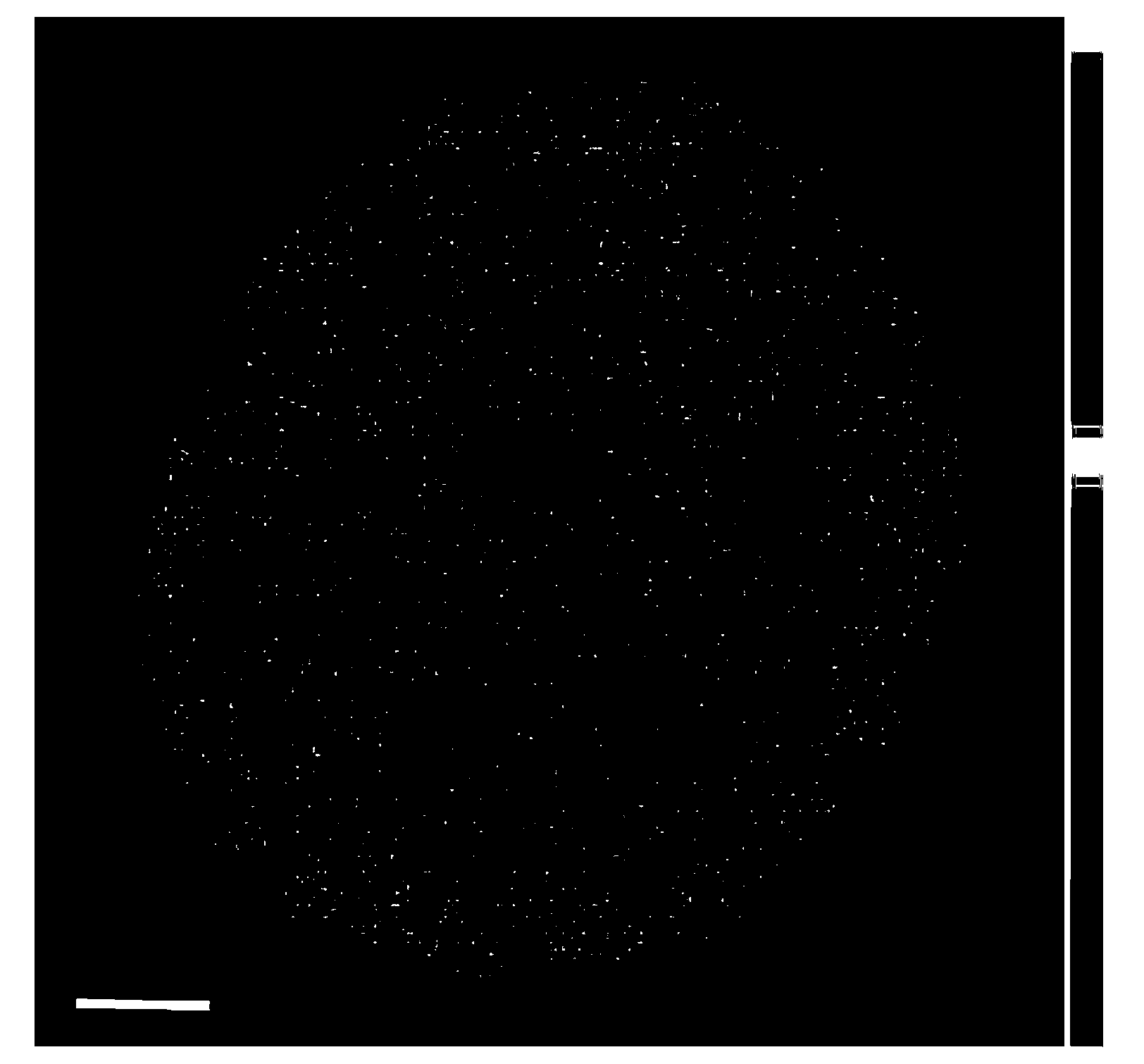
[0024] 2.56g PbCl 2 After dissolving with deionized water, add it to the alumina carrier, let it stand still, and then put it into a vacuum drying oven to dry for 12 hours. Calcined at 500°C for 3h in air atmosphere. Put the impregnated-calcined lead-containing alumina carrier into a 500mL beaker, add deionized water, and add 9.0mL H 2 PdCl 4 solution. At 25°C, add 30mL NaBH dropwise 4 solution, the reaction stopped after 10 h. Filter with suction, wash with plenty of water, and dry in a vacuum oven at 80°C for 12 hours. Gained catalyst is counted as: Pd 2 Pb 8 / Al 2 o 3 . The distribution of Pd on the alumina support is shown in figure 1 , it can be seen that Pd in the catalyst prepared by the new method is enriched in the "eggshell" region 20 μm below the outer surface of the alumina microspheres. The contents of Pd and Pb in the catalyst were measured by ICP-AES, see Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com