Preparation method of zinc silicate nanometer material
A nanomaterial, zinc silicate technology, applied in nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve problems such as unfavorable industrial production, increase preparation cost, etc., and achieve low reaction temperature, easy control of product composition, and size distribution. uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A preparation method of zinc silicate nanomaterials, using amorphous nano silicon dioxide and soluble zinc salt as raw materials, specifically comprises the following steps:
[0027] Step A, at room temperature, weigh 2mmol of amorphous nano-SiO with a diameter of about 200nm 2 Add to 10mL 0.4mol / L Zn(NO 3 ) 2 ·6H 2 O solution, stirred for 10 min to form a suspended aqueous solution, then adjusted the pH value of the reaction system to 5 with 1mol / L NaOH solution, and continued to stir for 30 min;
[0028] Step B, transfer the suspended aqueous solution in step A into a 25 mL hydrothermal reaction kettle, seal it and place it in a constant temperature box, and conduct a hydrothermal reaction at 220°C for 24 hours;
[0029] Step C, after the reaction is completed, the reaction system is cooled to room temperature, the reaction product is centrifuged, and then the reaction product is washed with deionized water and absolute ethanol for 5 times to remove impurity ions, ...
Embodiment 2
[0034] Step A, at room temperature, weigh 2mmol of amorphous nano-SiO with a diameter of about 200nm 2 Add to 10 mL 0.4 mol / L Zn(NO 3 ) 2 ·6H 2 O solution, stir 10min, form suspended aqueous solution, after the pH value of reaction system is adjusted to 9 with the NaOH solution of 1mol / L then, continue stirring 30min;
[0035]Step B, transfer the suspended aqueous solution in step A into a 25 mL hydrothermal reaction kettle, seal it and place it in a constant temperature box, and conduct a hydrothermal reaction at 220°C for 24 hours;
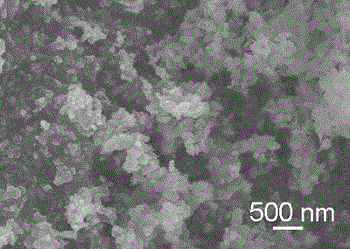
[0036] Step C, after the reaction is completed, the reaction system is cooled to room temperature, the reaction product is centrifuged, and then the reaction product is washed with deionized water and absolute ethanol for 5 times to remove impurity ions, and then the washed product is vacuum-dried Vacuum drying at 60°C for 4 hours in the oven to obtain Zn 2 SiO 4 Nano stave.
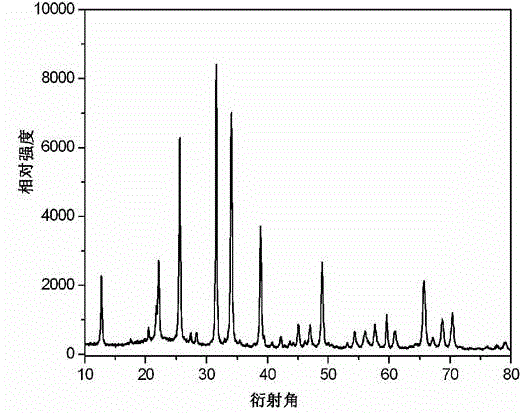
[0037] The Zn that present embodiment makes 2 SiO 4 The XRD s...
Embodiment 3
[0039] Step A, at room temperature, weigh 2mmol of amorphous nano-SiO with a diameter of about 200nm 2 Add to 10mL 0.4 mol / L Zn(NO 3 ) 2 ·6H 2 O solution, stirred for 20 min to form a suspended aqueous solution, then adjusted the pH value of the reaction system to 13 with 2mol / L NaOH solution, and continued to stir for 30 min;
[0040] Step B, transfer the suspended aqueous solution in step A into a 25 mL hydrothermal reaction kettle, seal it and place it in a constant temperature box, and conduct a hydrothermal reaction at 220°C for 24 hours;
[0041] Step C, after the reaction is completed, the reaction system is cooled to room temperature, the reaction product is centrifuged, and then the reaction product is washed with deionized water and absolute ethanol for several times to remove impurity ions, and then the washed product is placed in a vacuum drying oven Vacuum drying at 60°C for 4 hours to obtain Zn 2 SiO 4 Submicron rods.
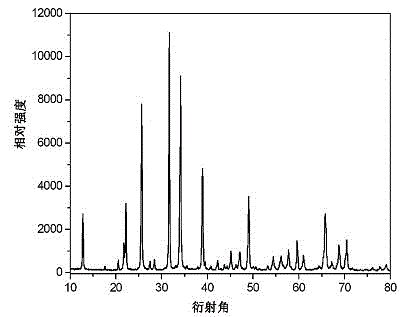
[0042] Zn prepared in this embodiment...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com