GS-DHFRmut double-gene screening expression vector, and preparation method and application thereof
A technology for gene expression and eukaryotic expression vector, which is applied in the field of GS-DHFRmut double gene screening expression vector and its preparation and application, and can solve the problems of reducing binding force and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
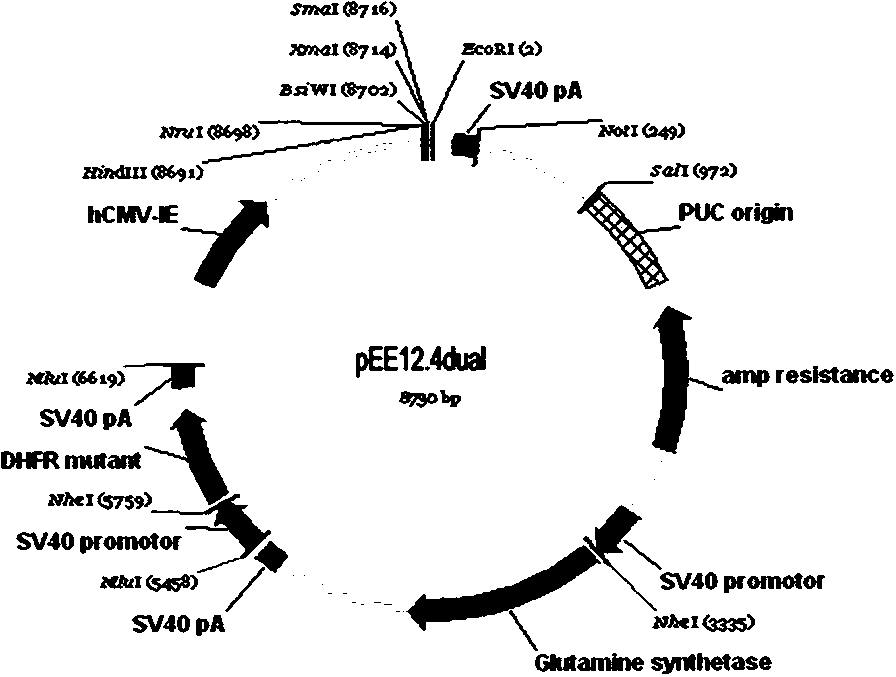
[0036] GS-DHFR mut Construction of double marker screening expression vector (pEE12.4dual): the DHFR mut The gene sequence was inserted into Lonza's commercial GS screening expression blank vector, pEE12.4.
[0037] DHFR mut Acquisition of the gene: According to the literature published in the scientific journal Biochemistry by Christlan C et al. in 1983, DHFR was confirmed mut The DNA sequence of the open reading frame (ORF), the specific sequence information is shown in SEQ No1. Then refer to the framework sequence of the commercial vector pEE12.4, in DHFR mut A promoter, PolyA signal sequence, and MluI restriction site are added upstream and downstream of the ORF to form a complete DHFR mut The gene sequence was synthesized by a gene synthesis company.
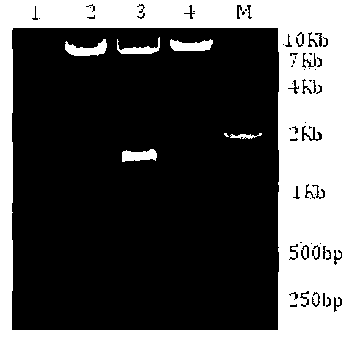
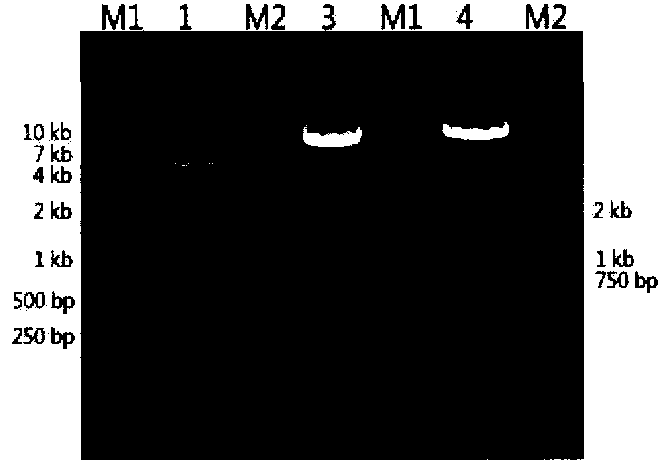
[0038] Single enzyme digestion: take the DHFR synthesized by entrusted gene company mut The gene and the commercial GS screening expression blank vector pEE12.4 purchased from Lonza Company were digested with Mlu I re...
Embodiment 2
[0046] GS-DHFR mut Construction of recombinant antibody expression vector for double-marker screening and GS single-marker screening for recombinant antibody expression vector.
[0047] Acquisition of recombinant monoclonal antibody mAb-1 gene: the amino acid sequence of the variable region (VH, VL) of the heavy chain and light chain of recombinant monoclonal antibody mAb-1 is derived from the patent WO-09630046 of Genentech in 1995, see SEQ No2 and No3; the amino acid sequence of the heavy chain constant region (CH) is derived from the sequence of the human IgG1 heavy chain constant region published on the protein database UniProt (query number P01857), see SEQ No4; the amino acid sequence of the light chain constant region (CL) is derived from protein The sequence of the human Ig kappa chain constant region (query number P01834) is published on the database UniProt, see SEQ No5. For the amino acid sequence of the obtained full-length recombinant monoclonal antibody mAb-1, t...
Embodiment 3
[0053] Electrotransfect the mAb1 gene expression vector and compare the expression levels of clones with different screening methods,
[0054] During the experiment, the expression vector for GS single-marker screening used the pEE12.4-6.4-mAb-1 vector constructed above; GS-DHFR mut The pEE12.4dual-6.4-mAb-1 constructed above was used as the dual screening expression vector.
[0055] 1. Cell culture and transfection
[0056] The day before electrotransfection, subculture the CHO K1 cells in suspension, the subcultured cell density is 0.5E6cell / ml, and the culture volume is 20ml CD CHO media (GIBCO). The next day, take 15E6 cells for electrotransfection, operate the electroporation instrument (Biorad) according to the operating instructions, and transfect plasmids A and B into CHOK1 cells; then dilute the electrotransfected CHOK1 cells with CD CHO medium, 20000cell / well; add the diluted cell suspension to 150ul / well of a 96-well plate. Cultivate at 37 degrees, 5% CO2, and u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com