Carbon monoxide methanating catalyst and preparation method thereof
A methanation catalyst, carbon monoxide technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, hydrocarbon production from carbon oxide, etc., can solve the problems of complex preparation process and high nickel content, Achieve high catalytic activity, concentrated pore size distribution, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
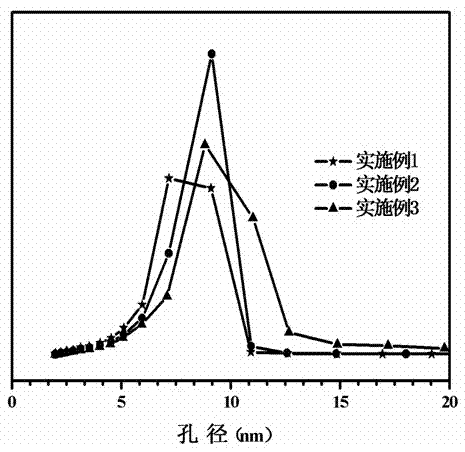
Embodiment 1
[0033] Weigh Ni(NO 3 ) 2 ·6H 2 O, Al(NO 3 ) 3 9H 2 O, Mn(NO 3 ) 2 , Zr(NO 3 ) 4 ·5H 2 O and dispersant A 3.613 g, 30.464 g, 1.112 g, 3.345 g, 0.3 g were made into a 200 mL solution, and sodium hydroxide was used as a precipitating agent, and co-current co-precipitation was carried out at 40 ° C, and the pH was controlled to be 8-10. Stirring was continued for 1 h after completion, and then left to age for 16 h. The resulting precipitate was filtered, dried at 90 °C for 12 h, and calcined at 700 °C for 4 h to obtain the catalyst.
Embodiment 2
[0035] Weigh Ni(NO 3 ) 2 6H 2 O, Al(NO 3 ) 3 9H 2 O, Fe(NO 3 ) 3 9H 2 O, Zr(NO 3 ) 4 5H 2 O and dispersant B 4.954g, 28.698g, 0.623g, 2.509g, 0.4g were made into a 200mL solution, and ammonium bicarbonate was used as a precipitating agent, and co-current co-precipitation was carried out at 60°C, and the pH was controlled to be 8-10. Stirring was continued for 1 h, and then aged for 16 h. The resulting precipitate was filtered, dried at 90°C for 12 h, and calcined at 800°C for 5 h to obtain the catalyst.
Embodiment 3
[0037] Weigh Ni(NO 3 ) 2 ·6H 2 O, Al(NO 3 ) 3 9H 2 O, Co(NO 3 ) 2 ·6H 2 O, Zr(NO 3 ) 4 ·5H 2 O and dispersant C 4.011 g, 30.464 g, 0.446 g, 0.836 g, 0.5 g were prepared into a 200 mL solution, and sodium carbonate was used as a precipitating agent, and co-current co-precipitation was carried out at 80°C, and the pH was controlled at 8-10, and the Stirring was continued for 1 h, and then aged for 16 h. The resulting precipitate was filtered, dried at 90°C for 12 h, and calcined at 800°C for 6 h to obtain the catalyst.
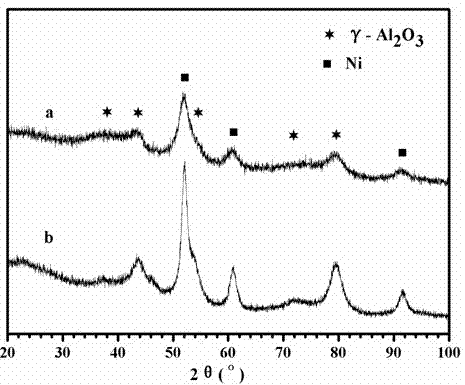
[0038] figure 1 It is the X-ray diffraction pattern after reduction of the catalyst (a) obtained in Example 2 of the present invention and the catalyst (b) obtained in Comparative Example 1. Depend on figure 1It can be seen that the catalyst designed in the present invention has no new diffraction peaks after adding additives, and the shape of each diffraction peak becomes diffuse, which promotes the dispersion of each component, and the grain size...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com