Medicinal bulk drug for resisting tumors
A technology of raw materials and medicinal salts, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Embodiment 1: Preparation of eribulin / eribulin mesylate bulk drug
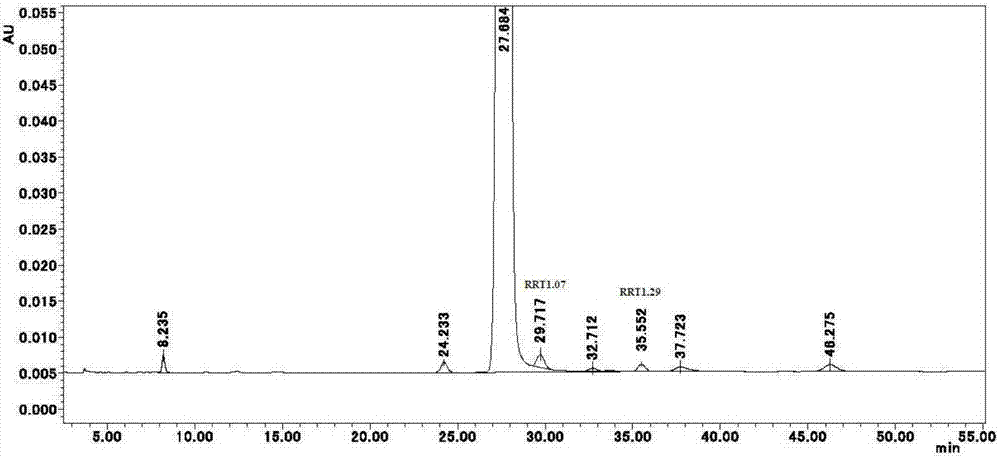
[0168] Step (1)——synthesis of the compound of formula I: According to CN1216051C (Chinese patent ZL99809658.X) instruction page 28, line 11, starting from L-arabinose synthesis triol 1, to instruction page 62, line 7, to obtain compound B1939, which is the formula I of the present invention Compound (free base form), the product obtained is a compound of formula I (crude product). It was determined by 【HPLC-A】, RRT1.07 content = 4.17%, RRT1.29 content = 0.73%.
[0169] Step (2)——salt formation: at a temperature of 20 to 25°C, add 50 grams of the product obtained in step (1) and 70 mmol of methanesulfonic acid to a mixed solution of 1400 ml of water and 1300 ml of acetonitrile, stir to react, and react The product was concentrated under reduced pressure; the resulting residue was dissolved in 400ml of dichloromethane, filtered, and 3000ml of n-pentane was added to the filtrate; the precipitate was fil...
Embodiment 2
[0171] Embodiment 2: Preparation of eribulin / eribulin mesylate bulk drug
[0172] Take the product obtained in step (2) of Example 1, and carry out recrystallization and purification with reference to the operation mode of step (3) of Example 1.
[0173] At a temperature of 30-35°C, dissolve 1.0 g of the product obtained in step (2) of Example 1 in 10 ml of anhydrous ethanol-ethyl acetate mixed solvent (1:3, v / v), and slowly add 60ml of n-hexane, let it stand still to allow sufficient precipitation (about 6 hours), filter out the precipitate, wash with n-hexane, and vacuum-dry to obtain (recrystallization yield 91.4%). It was determined by 【HPLC-A】, RRT1.07 content = 0.76%, RRT1.29 content = 0.33%. After one recrystallization purification, the content of RRT1.07 was reduced by 77.4%. It can be used as the eribulin mesylate bulk drug of the present invention.
Embodiment 3
[0174] Embodiment 3: Preparation of eribulin / eribulin mesylate bulk drug
[0175] Take the product obtained in step (2) of Example 1, and carry out recrystallization and purification with reference to the operation mode of step (3) of Example 1.
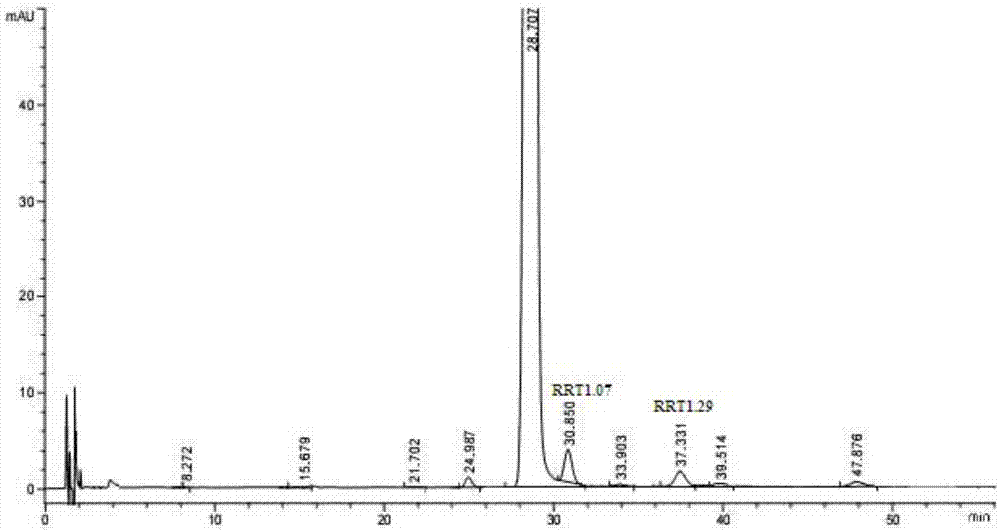
[0176] At a temperature of 35-40°C, 2.0 g of the product obtained in step (2) of Example 1 was dissolved in 10 ml of anhydrous ethanol-ethyl acetate mixed solvent (1:7, v / v), and slowly added dropwise 120ml of n-hexane, let it stand still to allow sufficient precipitation (about 36 hours), filter out the precipitate, wash with n-hexane, and vacuum-dry to obtain (recrystallization yield 95.2%). It was determined by [HPLC-A], RRT1.07 content = 0.91%, RRT1.29 content = 0.44%. After one recrystallization purification, the content of RRT1.07 was reduced by 72.9%. It can be used as the eribulin mesylate bulk drug of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com