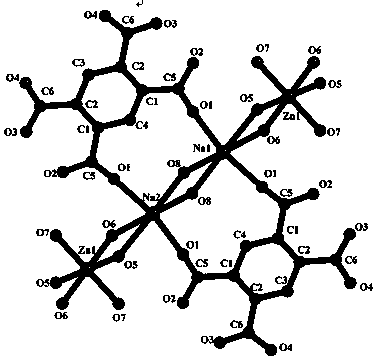
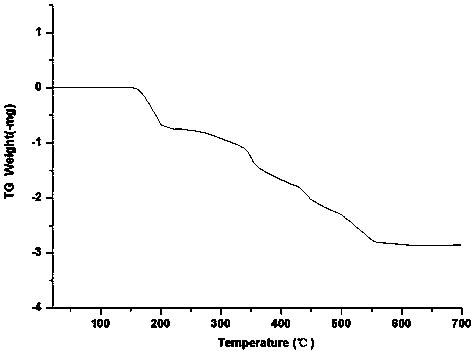
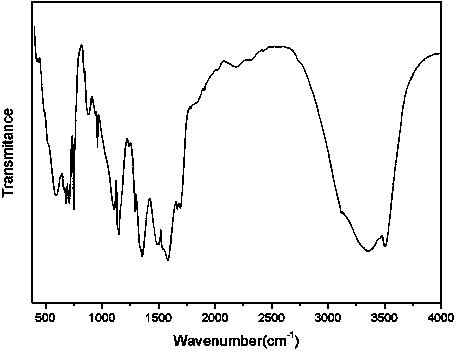
Organic complex with metal zinc as center body, preparation method thereof and application thereof
A metal complex, metal-organic technology, applied in organic chemistry, chemical instruments and methods, other chemical processes, etc., can solve the problems of increased diffusion process, low yield, and lack of good crystal structure, and achieve a synthesis method. Simple, productive and reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] First, put 0.508g (2mmol) of pyromellitic acid, 0.595g (2mmol) of zinc nitrate hexahydrate (2mmol) and 0.200g (5mmol) of sodium hydroxide into three dry 50ml beakers, respectively, with 3ml water, 5ml water and Dissolve in 5ml of water and stir thoroughly for 15 minutes to completely dissolve the solid in the solvent;
[0040] Second, under stirring conditions, the sodium hydroxide solution was added dropwise to the pyromellitic acid solution for 5 minutes, and then the zinc nitrate hexahydrate solution was poured into the mixed solution;
[0041] The 3rd, mixed solution is all transferred in the 25ml hydrothermal reaction kettle, puts into electric blast drying box, setting temperature is 150 DEG C, rises to 150 DEG C from room temperature with the time of 12 hours (half a day), and Keep it for 4 days, then cool it down to room temperature in 2 days, that is, the program is 0.5-4-2;
[0042] Finally, the reacted solution is taken out and filtered to obtain the che...
Embodiment 2
[0044] First, put 0.508g (2mmol) of pyromellitic acid, 0.892g (3mmol) of zinc nitrate hexahydrate (3mmol) and 0.240g (6mmol) of sodium hydroxide into three dry 50ml beakers, respectively, with 5ml water, 9ml water and Dissolve in 6ml of water and stir thoroughly for 20 minutes to completely dissolve the solid in the solvent;
[0045] Second, under stirring conditions, the sodium hydroxide solution was added dropwise to the pyromellitic acid solution for 7 minutes, and then the zinc nitrate hexahydrate solution was poured into the mixed solution;
[0046] The 3rd, mixed solution is all transferred in the 25ml hydrothermal reaction kettle, puts into electric blast drying box, setting temperature is 160 DEG C, rises to 160 DEG C from room temperature with the time of 12 hours (half a day), and Keep it for 4 days, then cool it down to room temperature in 2 days, that is, the program is 0.5-4-2;
[0047] Finally, the reacted solution is taken out and filtered to obtain the chemica...
Embodiment 3
[0049] First, put 0.508g (2mmol) of pyromellitic acid, 1.190g (4mmol) of zinc nitrate hexahydrate (4mmol) and 0.280g (7mmol) of sodium hydroxide into three dry 50ml beakers, respectively, with 6ml water, 10ml water and Dissolve in 8ml of water and stir thoroughly for 30 minutes to completely dissolve the solid in the solvent;
[0050] Second, under stirring conditions, the sodium hydroxide solution was added dropwise to the pyromellitic acid solution for 10 minutes, and then the zinc nitrate hexahydrate solution was poured into the mixed solution,
[0051] The 3rd, mixed solution is all transferred in the 25ml hydrothermal reaction kettle, puts into electric blast drying box, set temperature is 170 DEG C, rises to 170 DEG C from room temperature with the time of 12 hours (half a day), and Keep it for 4 days, then cool it down to room temperature in 2 days, that is, the program is 0.5-4-2;
[0052] Finally, the reacted solution is taken out and filtered to obtain the chemical ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com