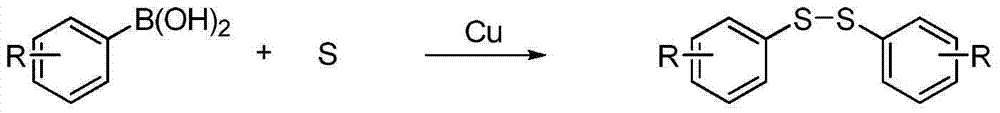
Synthetic method for symmetrical diaryl disulfide
A kind of diaryl disulfide, symmetrical technology, applied in the field of catalytic synthesis of diaryl disulfide, can solve the problems of complicated operation, no literature report, insufficient environmental protection and the like, and achieves the effect of simple product separation and simplified formation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The synthesis of embodiment 1 diphenyl disulfide
[0017]
[0018] Copper sulfate is used as the catalyst, the amount of catalyst is 5% of the moles of phenylboronic acid, the molar ratio of raw materials is phenylboronic acid: elemental sulfur 1:1.5, the solvent is N,N'-dimethylformamide, and the dosage is 2.5mL / mmol of benzene Boric acid, stirred and heated to 50°C, the reaction time was 5 hours. After the reaction, filter, extract with ethyl acetate, distill off the solvent under reduced pressure, and recrystallize the residue in ethanol to obtain a white solid product. The conversion of phenylboronic acid was 100%, and the yield of diphenyl disulfide was 95%.
Embodiment 2 2
[0019] The synthesis of embodiment 2 di-p-tolyl disulfide
[0020]
[0021] Copper sulfate is used as a catalyst, the amount of catalyst is 5% of the moles of 4-methylbenzeneboronic acid, the molar ratio of raw materials is 4-methylbenzeneboronic acid: elemental sulfur 1:1.5, solvent N,N'-dimethylformamide, The dosage is 2.5mL / mmol 4-methylphenylboronic acid, stirred and heated to 50oC, and the reaction time is 5 hours. After the reaction, filter, extract with ethyl acetate, distill off the solvent under reduced pressure, and recrystallize the residue in ethanol to obtain a white solid product. The conversion of phenylboronic acid was 100%, and the yield of di-p-tolyl disulfide was 97%.
Embodiment 3 2
[0022] The synthesis of embodiment 3 two (2-methylphenyl) disulfides
[0023]
[0024] Copper sulfate is used as a catalyst, the amount of catalyst is 5% of the molar number of 2-methylbenzeneboronic acid, the molar ratio of raw materials is 2-methylbenzeneboronic acid: elemental sulfur 1:1.5, solvent N,N'-dimethylformamide, The dosage is 2.5mL / mmol of 2-methylphenylboronic acid, stirred and heated to 60°C, and the reaction time is 7 hours. After the reaction, filter, extract with ethyl acetate, distill off the solvent under reduced pressure, and recrystallize the residue in ethanol to obtain a white solid product. The conversion of 2-methylphenylboronic acid was 100%, and the yield of bis(2-methylphenyl)disulfide was 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com