Single-dosage gatifloxacin eye drop and preparation method thereof
A technology of gatifloxacin and eye drops, applied in pharmaceutical formulations, drug delivery, sensory diseases, etc., can solve the problems of ophthalmic preparations judgment standards, waste of resources, narrow antibacterial spectrum, etc., to avoid toxic side effects and potential risk, avoid potential hazards, and save costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1. Single-dose gatifloxacin eye drops, without bacteriostatic agent, for one-time use, the volume of the individually packaged medicine solution is 0.4mL / bottle, and the content of gatifloxacin is 0.5% (g / mL).
[0067] prescription:
[0068]
[0069]
[0070] A total of 2,500 pieces were made.
[0071] Preparation:
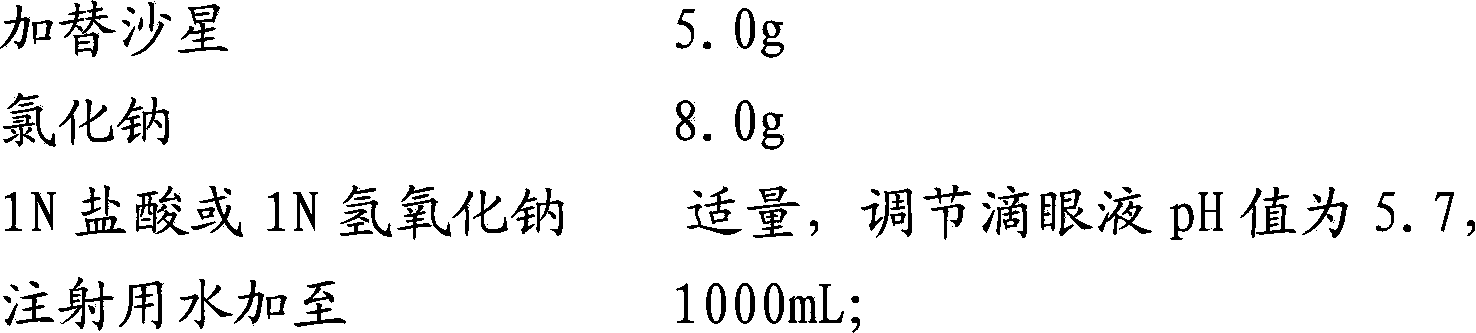
[0072] a. Accurately weigh 5.0g of gatifloxacin, add it to 200mL of 0.1mol / L hydrochloric acid to fully dissolve, add water for injection to 80% of the total volume; accurately weigh 8.0g of sodium chloride, and add it to the above solution; use 1N Adjust the pH value to 5.7 with hydrochloric acid or 1N sodium hydroxide solution, and make up the prescribed amount of water for injection to obtain a medicinal solution;
[0073] b. Filter the medicinal solution obtained in step a through a 0.22 μm microporous membrane for 2 times;
[0074] c. Fill 0.4mL of liquid medicine into a 1mL single-dose packaging container in a class A clean area, and ...
Embodiment 2
[0076] Example 2. Single-dose gatifloxacin eye drops, free of bacteriostatic agents, for one-time use, individually packaged with a volume of 0.6 mL / bottle and a content of 0.5% (g / mL).
[0077] prescription:
[0078]
[0079] A total of 1666 pieces were made.
[0080] Preparation:
[0081] a. Accurately weigh 5.0g of gatifloxacin, add it to 200mL of 0.1mol / L hydrochloric acid to fully dissolve, add water for injection to 80% of the total volume; accurately weigh 6.39g of sodium dihydrogen phosphate and 2.89g of disodium hydrogen phosphate 12.0 g of glycerol is added in the above-mentioned solution; the pH value is adjusted to 6.0 with 1N hydrochloric acid or 1N sodium hydroxide solution, and the water for injection of the prescribed amount is supplemented to obtain a medicinal solution;
[0082] b. Filter the medicinal solution obtained in step a through a 0.22 μm microporous membrane for 2 times;
[0083] c. Fill 0.6mL of liquid medicine into a 1mL single-dose packagin...
Embodiment 3
[0085] Example 3. Single-dose gatifloxacin eye drops, free of bacteriostatic agents, for one-time use, individually packaged with a volume of 0.8mL / bottle and a content of 0.5% (g / mL).
[0086] prescription:
[0087]
[0088] A total of 1250 pieces were made,
[0089] Preparation:
[0090] a. Accurately weigh 5.0g of gatifloxacin, and add it into 200mL of 0.1mol / L hydrochloric acid to fully dissolve; accurately weigh 12.0g of boric acid, 1.0g of borax, and 2.0g of sodium chloride, and add it into the above solution; or 1N sodium hydroxide solution to adjust the pH value to 8.0, and make up the prescribed amount of water for injection to obtain a medicinal solution;
[0091] b. Filter the medicinal solution obtained in step a through a 0.22 μm microporous membrane for 2 times;
[0092] c. Fill 0.8mL of liquid medicine into a 1mL single-dose packaging container in a class A clean area, seal the seal, and obtain the finished product.
[0093] Use the osmotic pressure detec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com