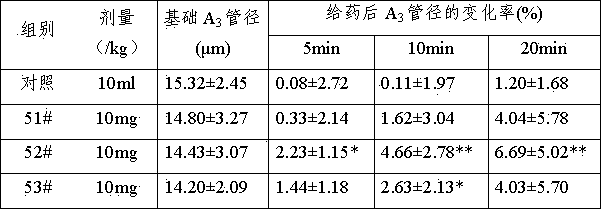
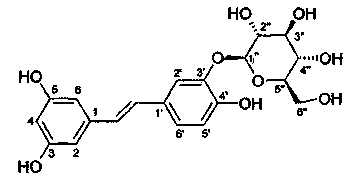
Application of 3,5,4'-trihydroxy-stilbyl-3'-O-glucoside in preparation of microcirculatory disturbance improvement medicines
A technology of microcirculation disorder and tristilbene, which is applied in the field of application of tristilbene in the preparation of drugs for improving blood microcirculation disorder, can solve the problems of insignificant clinical treatment effect, large side effects, poor curative effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] formula 1 2 3 4 5 Tristilbene 5g 5g 5g 10g 20g gastrodin 0.5g 80g 400g 80g 200g sodium hydroxide or hydrochloric acid Appropriate amount Appropriate amount Appropriate amount Appropriate amount Appropriate amount Water for Injection 1000ml 1000ml 1000ml 1000ml 1000ml
[0028] Add 60% of the prepared water for injection into the preparation tank, heat to 95°C and slightly boil, keep warm to 80°C, start the vacuum and start stirring for 10 minutes at the same time, use nitrogen gas to bring the tristilbene glycoside and gastrodin together according to the prescription amount Put it into the preparation tank, dissolve tristilbeside in about 5 minutes, remove the vacuum and nitrogen, adjust the pH to 5.0-7.0 and add water to the full amount to complete the preparation process, followed by sterile filtration, filling, and freeze-drying according to the conventional process made into injections.
Embodiment 2
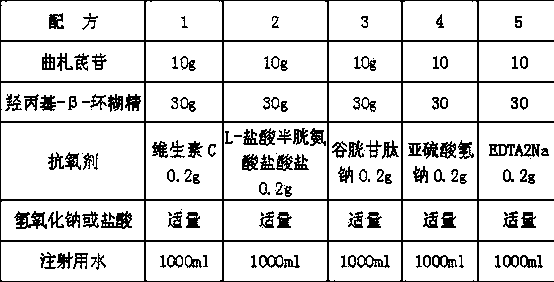
[0030] formula 1 2 Tristilbene 25g 50g gastrodin 80g 100g Hydroxypropyl-β-cyclodextrin 30g 30g sodium hydroxide or hydrochloric acid Appropriate amount Appropriate amount Water for Injection 1000ml 1000ml
[0031] Add 60% of the water for injection in the preparation tank, heat to 95°C and slightly boil, keep warm to 80°C, start the vacuum and stir for 10 minutes at the same time, use nitrogen gas to weigh the tristilbene, gastrodin, and hydroxy Propyl-β-cyclodextrin is brought into the preparation tank together, about 5 minutes to dissolve tristilbene, remove the vacuum and nitrogen, adjust the pH to 6.0-7.0 and add water to the full amount to complete the preparation process, and then follow the routine The process is aseptic filtration, filling, and freeze-drying to make injections.
Embodiment 3
[0033] formula 1 2 3 4 Tristilbene 5g 10g 10g 20g Hydroxypropyl-β-cyclodextrin 30g 30g 50g 50g sodium hydroxide or hydrochloric acid Appropriate amount Appropriate amount Appropriate amount Appropriate amount Water for Injection 1000ml 1000ml 1000ml 1000ml
[0034] Add 60% of the prepared water for injection into the preparation tank, heat to 95°C for a slight boil, keep warm to 80°C, start the vacuum and stir for 10 minutes at the same time, use nitrogen gas to weigh the tristilbene and hydroxypropyl- Bring β-cyclodextrin into the preparation tank together, and it will dissolve tristilbene in about 5 minutes. Remove the vacuum and nitrogen, adjust the pH to 5.5-7.0 and add water to the full amount to complete the preparation process, and then follow the normal process of aseptic Filtration, filling, and freeze-drying to make injections.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com