Method for depolymerizing lignin into aromatic compounds under conditions of no additional hydrogen
A technology for aromatic compounds and lignin, applied in organic chemistry, ether preparation, etc., can solve the problems of high risk factor and harsh conditions, and achieve the effect of high safety factor, mild reaction conditions and good atom economy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
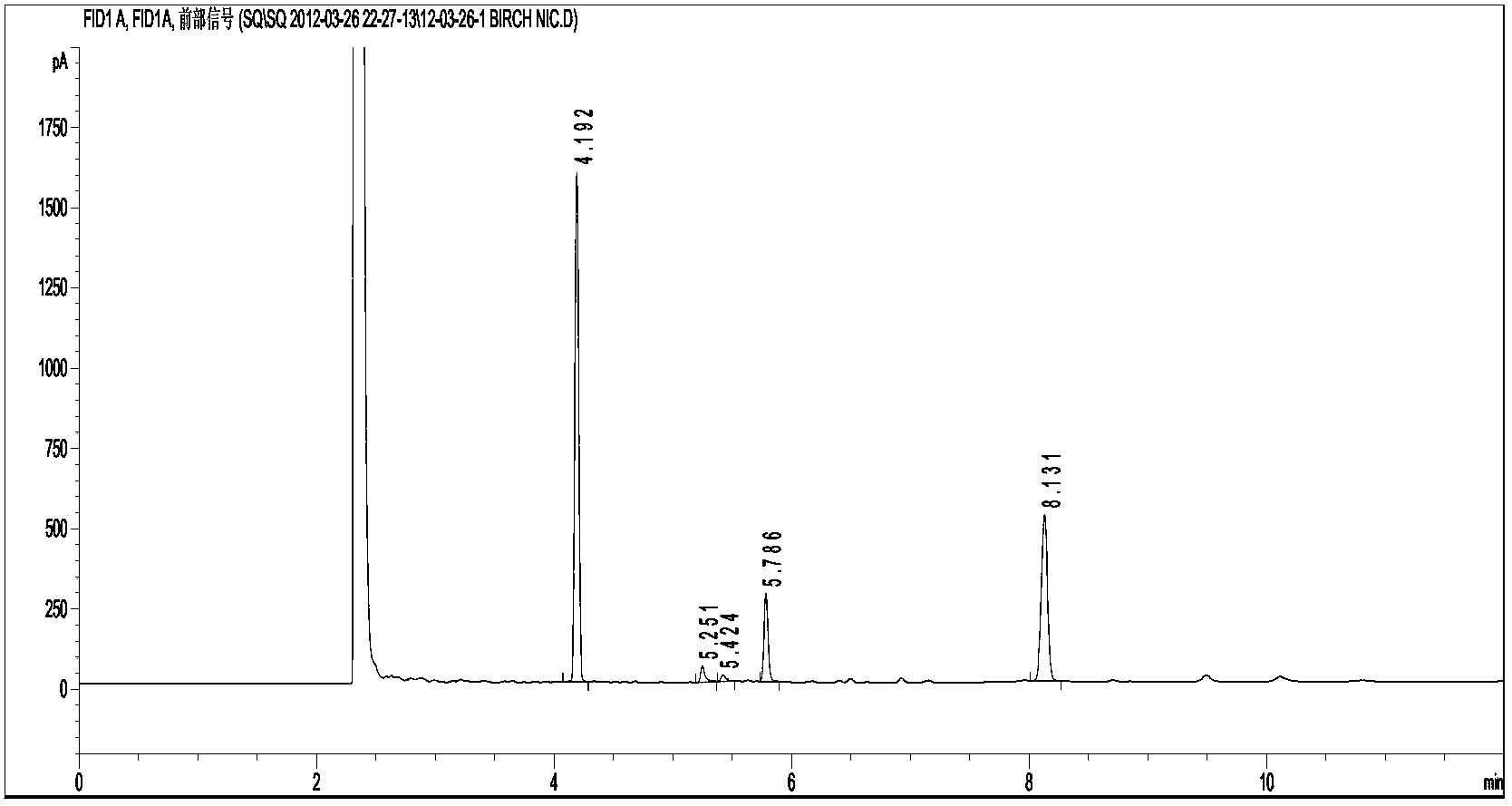
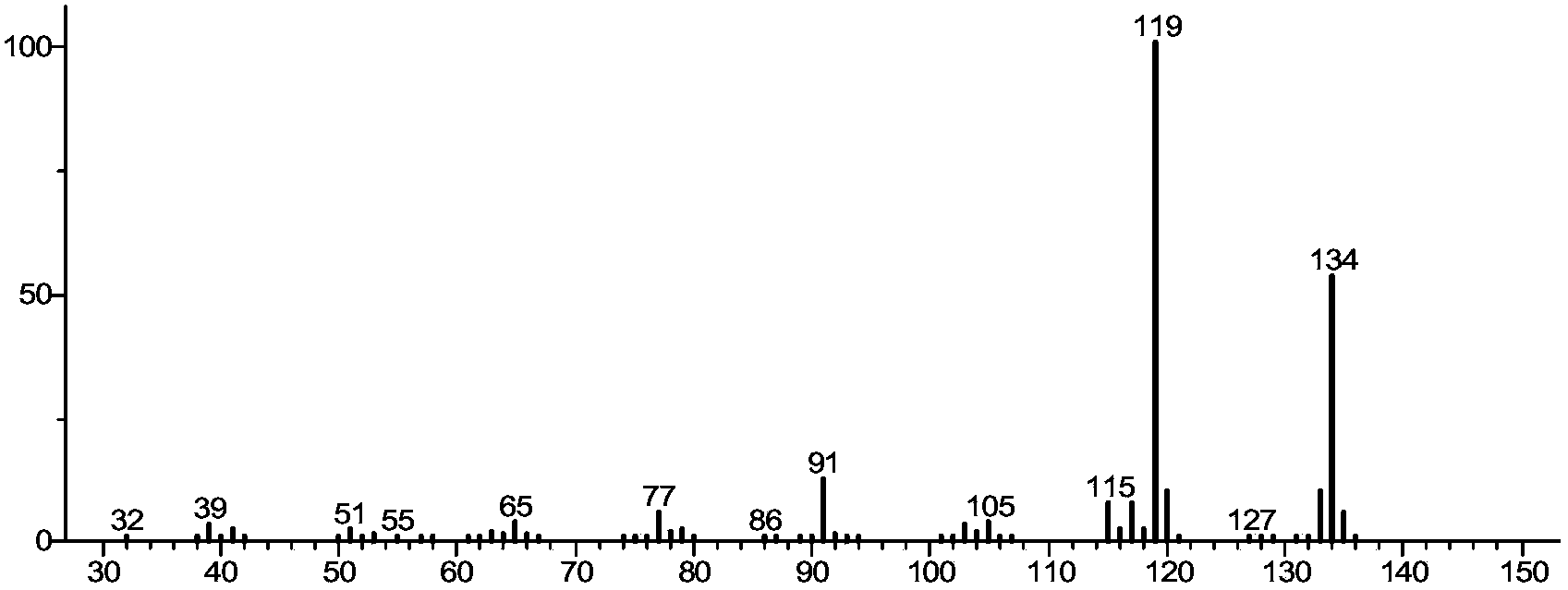
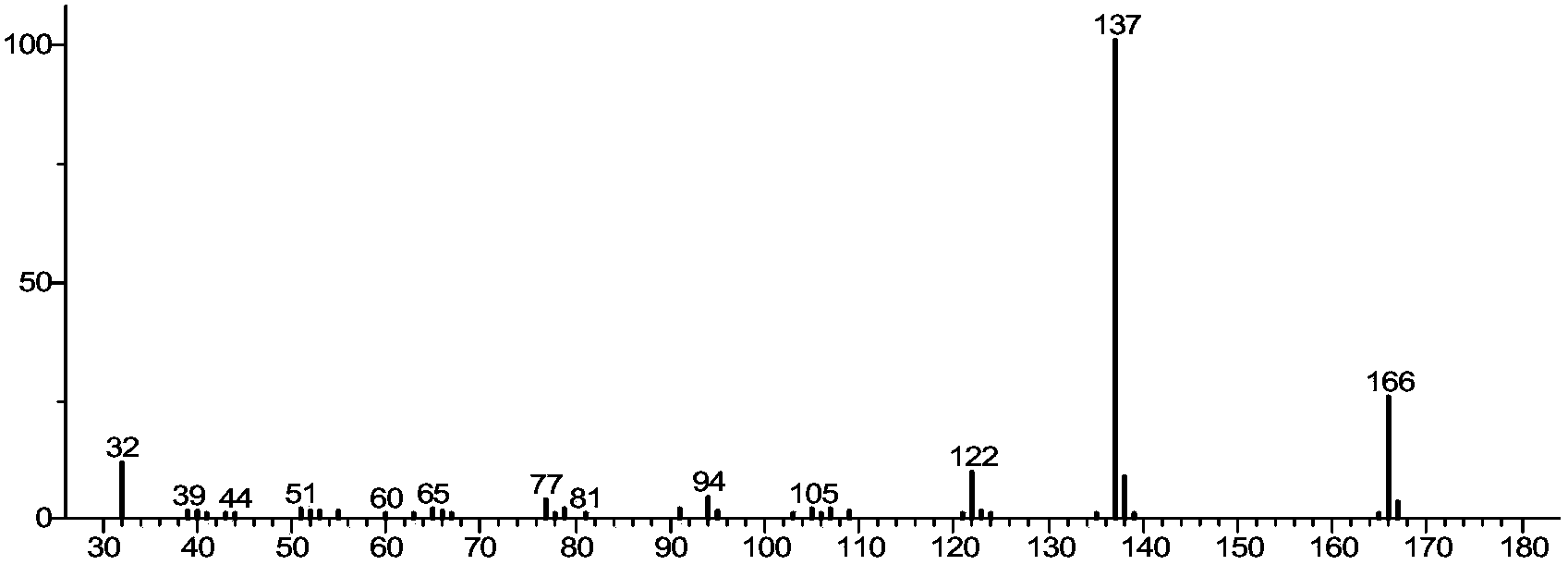
Image
Examples
Embodiment 1
[0026] Weigh 1 gram of nickel nitrate and dissolve it in 100 milliliters of water, add dried activated carbon (according to the loading amount of Ni is 8wt%), impregnate for 24 hours, and dry for 12 hours. Then, under the protection of nitrogen, calcined at 400° C. for 3 hours in a quartz tube. Afterwards hydrogen reduction was performed at 450 °C for 3 h. As the precursor of the metal component of the catalyst, metal nitrates are used for Ni, Al, Co, La, Ce, Cr, Zn, Fe, Cu and other metal salts, ammonium molybdate is used for Mo salt, and metal chloride salt is used for Mn and Sn salts.
Embodiment 2
[0028] Weigh 1 gram of nickel nitrate and dissolve it in 100 milliliters of water, add dried activated carbon (according to the loading amount of Ni is 5 wt%), impregnate for 24 hours, and dry for 12 hours. Then, under the protection of nitrogen, it was reduced by activated carbon at 450°C in a quartz tube for 3 hours.
Embodiment 3
[0030] The preparation process of the catalyst was carried out by the method of Example 1 (marked as a) or Example 2 (marked as b), except that different metal components and mass ratios and different supports were changed.
[0031] See Table 1 for details.
[0032] Table 1
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com