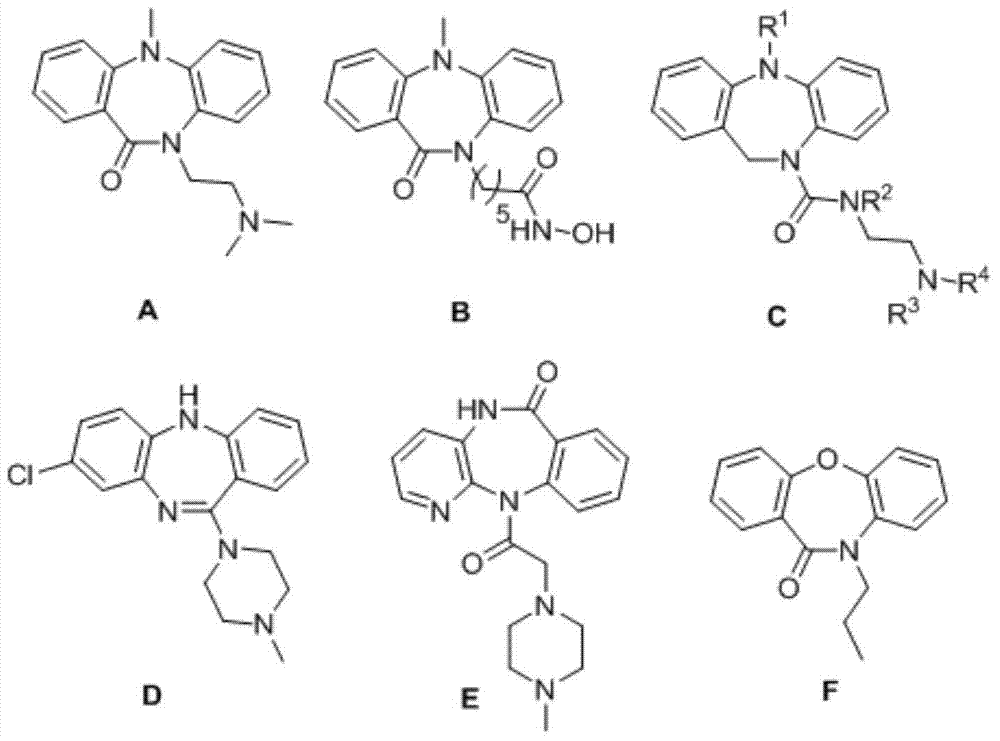
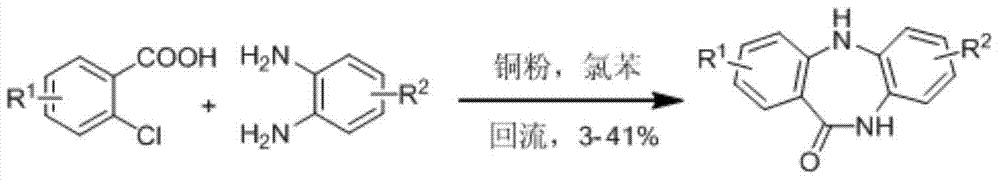
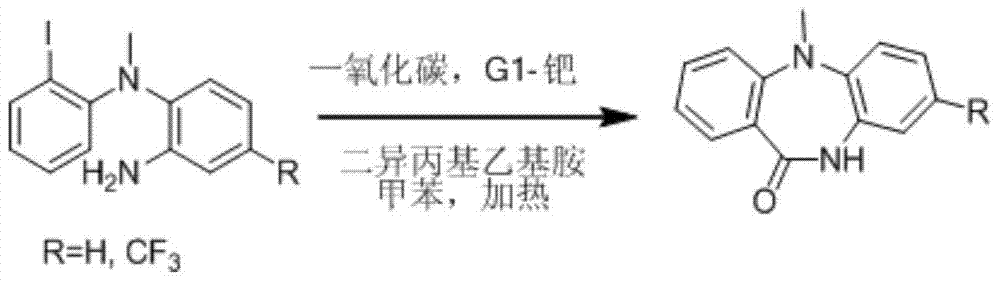
Synthetic method for dibenzepin derivative
A technology of dibenzepines and a synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of inconvenient handling of metal catalysts, environmental pollution and the like, and achieve the effects of simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A method for synthesizing diphenazepine derivatives (I), comprising the steps of:
[0048] (1) Preparation of 2-(N-methyl-N-phenyl)amino-N’-methoxybenzamide (VII-a)
[0049]Add o-bromobenzoic acid (II-a) (4.00 g), aniline (III-a) (2.79 g), nitrogenmethylmorpholine (3.3 mL), cuprous oxide (1.43 g) to dioxane ( 50 mL), heated to reflux for 4 hours under nitrogen protection. After the reaction was completed, cool to room temperature, remove the solid residue by suction filtration under reduced pressure, acidify the filtrate with 1M aqueous hydrochloric acid to pH=5, and obtain a white solid as 2-(N-phenyl)aminobenzoic acid (IV-a) by suction filtration.
[0050] Compound (IV-a) was dissolved in N,N-dimethylformamide (40 mL), sodium hydride (0.96 g) was added under ice cooling, and stirred at room temperature for half an hour. Transfer to an ice bath, add iodomethane (3.7 mL) dropwise, and stir at 0°C for one hour. After the reaction is over, slowly add water in an ice ba...
Embodiment 2
[0058] A method for synthesizing diphenazepine derivatives (I), comprising the steps of:
[0059] (1) Preparation of 2-[N-methyl-N-(4-methoxy)phenyl]amino-N’-methoxybenzamide (VII-b)
[0060] Add o-bromobenzoic acid (II-a) (4.00g), 4-methoxyaniline (III-b) (3.69g), nitrogenmethylmorpholine (3.3mL), cuprous oxide (1.43g) to Dioxane (50 mL) was heated to reflux for 4 hours under the protection of nitrogen. After the reaction was completed, cool to room temperature, remove the solid residue by suction filtration under reduced pressure, acidify the filtrate to pH=5 with 1M aqueous hydrochloric acid solution, and obtain a white solid of 2-[N-(4-methoxy)phenyl]aminobenzene by suction filtration Formic acid (IV-b).
[0061] Compound (IV-b) was dissolved in N,N-dimethylformamide (40 mL), sodium hydride (0.96 g) was added under ice cooling, and stirred at room temperature for half an hour. Transfer to an ice bath, add iodomethane (3.7 mL) dropwise, and stir at 0°C for one hour. Aft...
Embodiment 3
[0069] A method for synthesizing diphenazepine derivatives (I), comprising the steps of:
[0070] (1) Preparation of 2-[N-methyl-N-(3-methoxy)phenyl]amino-N’-methoxybenzamide (VII-c)
[0071] Add o-bromobenzoic acid (II-a) (4.00 g), 3-methoxyaniline (III-c) (3.69 g), nitrogen methylmorpholine (3.3 mL), cuprous oxide (1.43 g) to Dioxane (50 mL) was heated to reflux for 4 hours under the protection of nitrogen. After the reaction, cool to room temperature, remove the solid residue by suction filtration under reduced pressure, the filtrate is adjusted to pH=5 with 1M hydrochloric acid aqueous solution, and the white solid is 2-[N-(3-methoxy)phenyl]aminobenzene by suction filtration Formic acid (IV-c).
[0072] Compound (IV-c) was dissolved in N,N-dimethylformamide (40 mL), sodium hydride (0.96 g) was added under ice cooling, and stirred at room temperature for half an hour. Transfer to an ice bath, add iodomethane (3.7 mL) dropwise, and stir at 0°C for one hour. After the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com