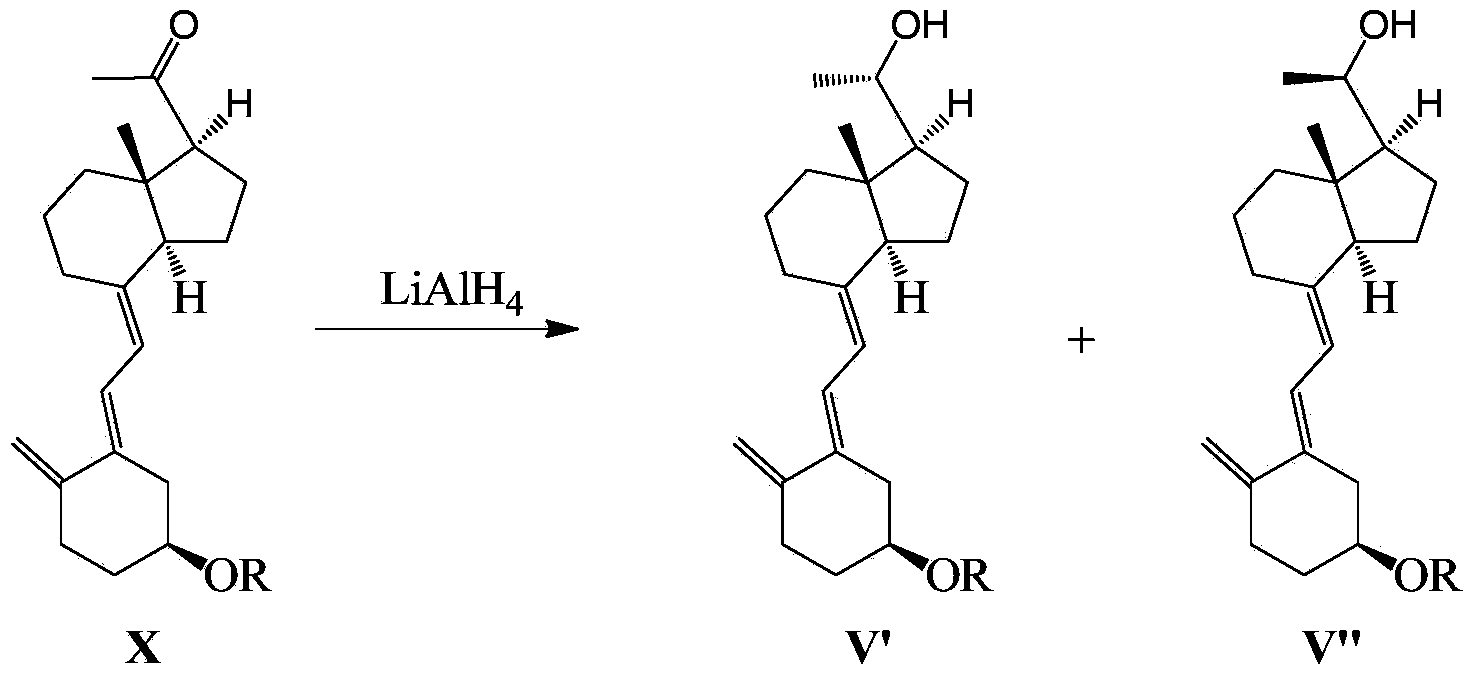
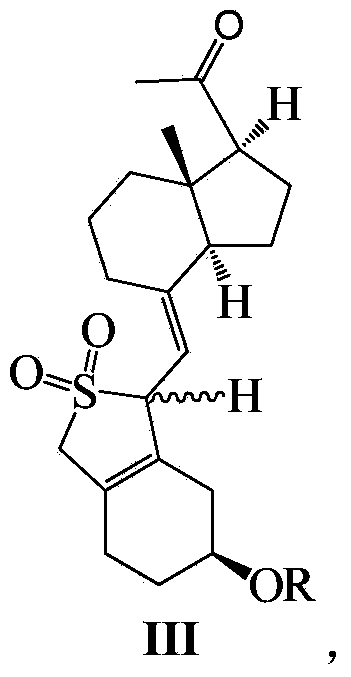
Maxacalcitol synthesizing intermediate and preparation method and application thereof
A technology of maxacalcitol and compounds, which is applied in the field of drug synthesis and can solve problems such as low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Preparation of compound III-1
[0121]
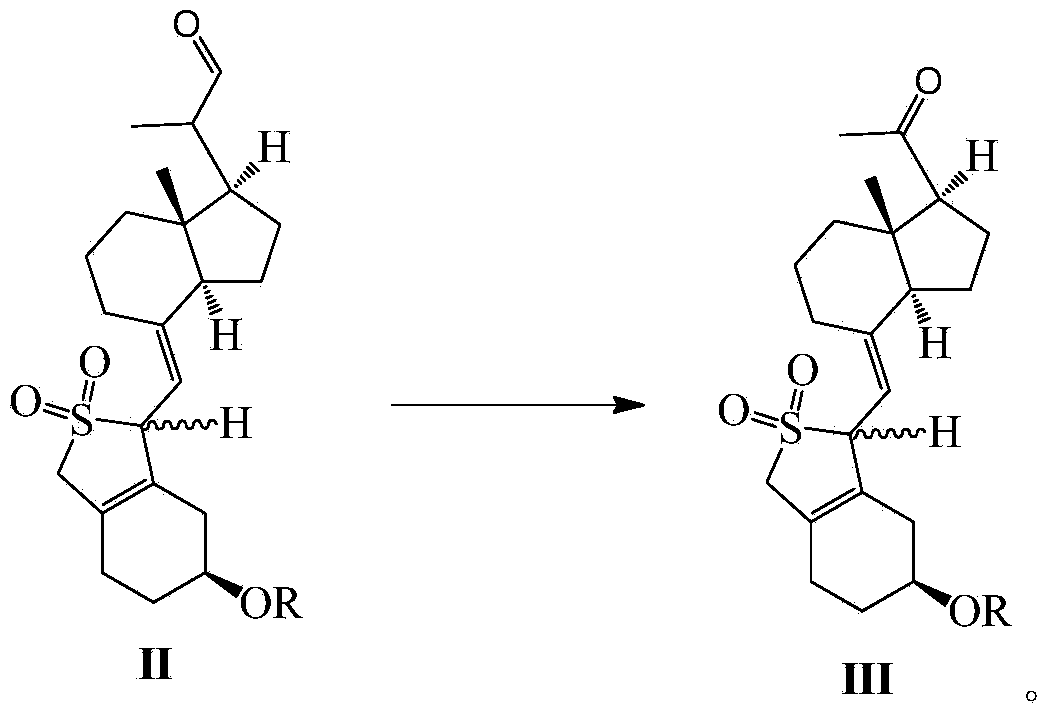
[0122] Compound II-1 (50.7g, 100mmol) was dissolved in DMF (500mL), and triethylenediamine (11.2g, 100mmol), 2,2-bipyridine (3.12g, 20mmol) and copper acetate (3.64 g, 20mmol). After the addition was complete, it was heated to 45°C under an oxygen atmosphere, and stirring was continued at this temperature for 5 hours. After the reaction was complete, ethyl acetate was added, and the insoluble matter was removed by suction filtration. The filtrate was washed 3 times with water. After drying over anhydrous sodium sulfate and concentrating under reduced pressure, the resulting oil was separated and purified to obtain Compound III-1 (39.9 g, yield 81%). This compound is a mixture of two configurations (caused by sulfur dioxide protection), which can be directly used in the next reaction. After a small amount of separation and purification, a compound of configuration 1 (large Rf value) and a compound of configuration 2 (small ...
Embodiment 2
[0127] Preparation of Compound IV-1
[0128]
[0129] Compound III-1 (49.2g, 100mmol) was dissolved in 400ml of anhydrous THF, and (R)-2-methyl-CBS-oxazoborin (1M, 100ml) was slowly added at -20oC, followed by Slowly add BH dropwise 3 ·THF solution (1M, 60ml), continue to stir for 1 hour after the addition, slowly rise to room temperature, add 50ml of saturated aqueous ammonium chloride solution, extract with ethyl acetate, concentrate to dryness under reduced pressure to obtain 49.5g of oil. The resulting oil is a mixture of two configurations (caused by sulfur dioxide protection, C-20 is a single S configuration). After a small amount of separation and purification, a compound of configuration 1 (large Rf value) and a compound of configuration 2 (small Rf value) were obtained.
[0130] The two isomers of compound IV-1 1 H NMR, 13 C NMR and MS measurement data are as follows:
[0131] Isomers with small Rf values: 1H NMR (400MHz, d-CHCl3) δ: -0.01and-0.00(each,s,6H),0...
Embodiment 3
[0134] Preparation of Compound V-1
[0135]
[0136] The crude compound IV-1 obtained in the previous step reaction was dissolved in 400ml of 95% ethanol, 50g of sodium bicarbonate was added under stirring, heated to reflux, and the reaction was continued at this temperature for 2 to 3 hours. After the reaction was complete, the ethanol was removed under reduced pressure and extracted with ethyl acetate. The resulting oil was separated and purified to obtain 36.4 g of compound V-1, with a yield of 84%.
[0137] Compound V-1 1 H NMR, 13 C NMR and MS measurement data are as follows:
[0138] 1 H NMR (400MHz, CDCl 3 )δ: -0.03(s,6H,2SiCH 3 ),0.50(s,3H,CH 3 ),0.82(s,9H,3SiCH 3 ),1.16(d,J=6Hz,3H,CH 3 ),1.18-1.23(m,2H),1.35-2.22(m,13H),2.38-2.43(m,1H),2.57-2.61(m,1H),2.79-2.83(m,1H),3.64-3.67 (m,1H,CHOH),3.78-3.81(m,1H,CHOH),4.58(s,1H,=CH 2 ),4.86(s,1H,=CH 2 ),5.81(d,J=11.6Hz,1H,=CH),6.40(d,J=11.6Hz,1H,=CH); 13 C NMR (75MHz, CDCl 3 )δ: -4.7, -4.6, 12.7, 18.2, 22.2, 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com