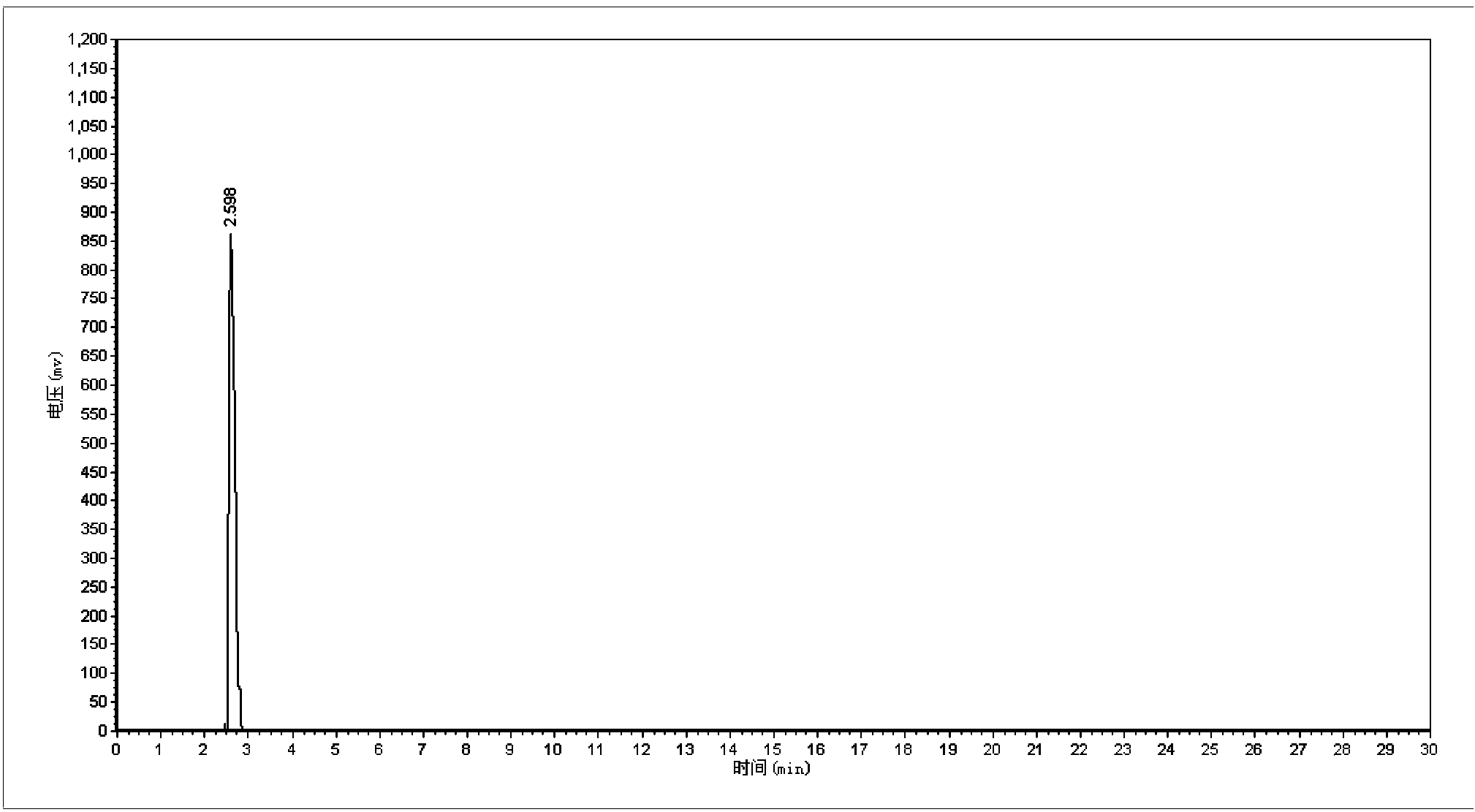
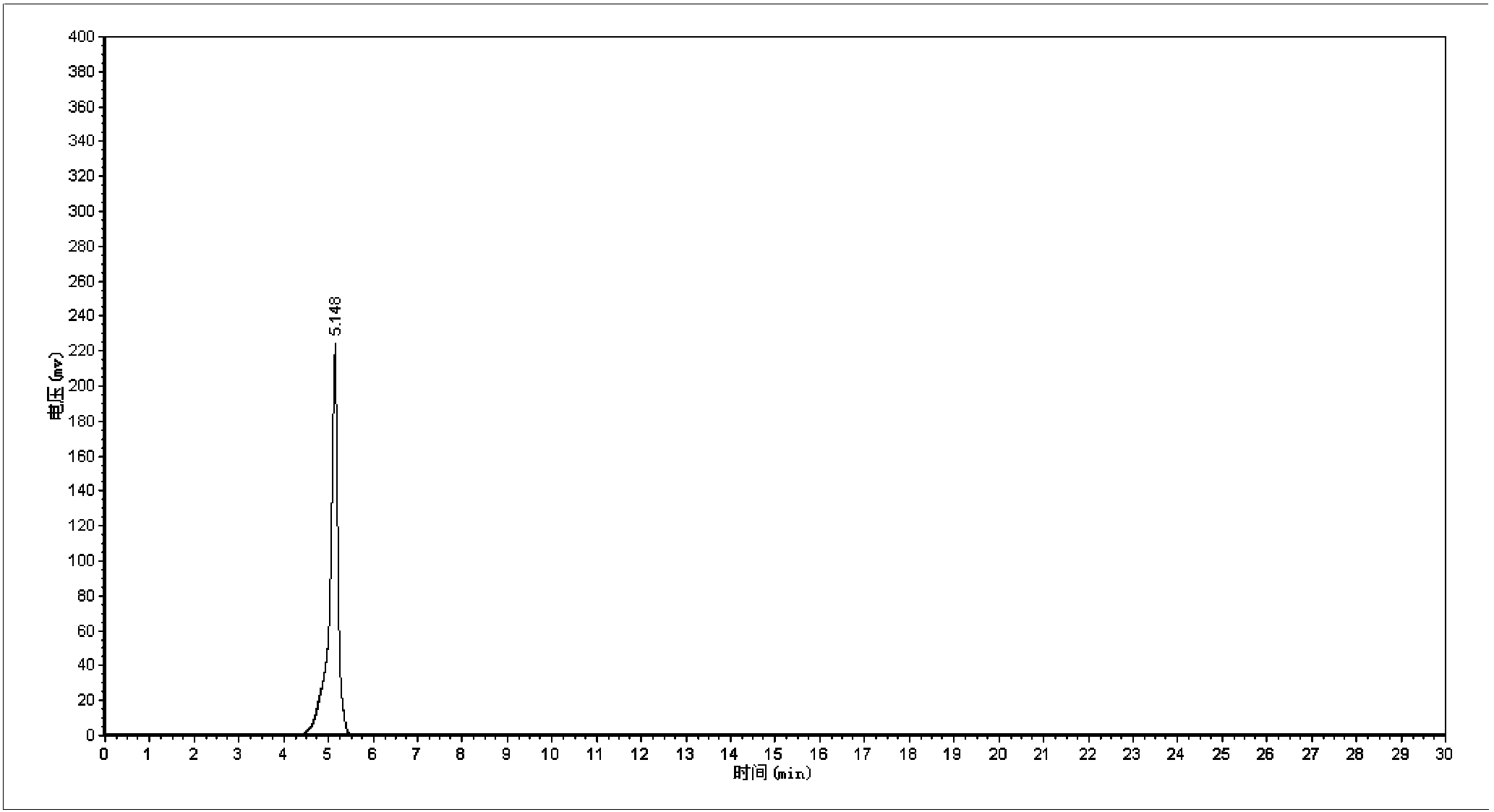
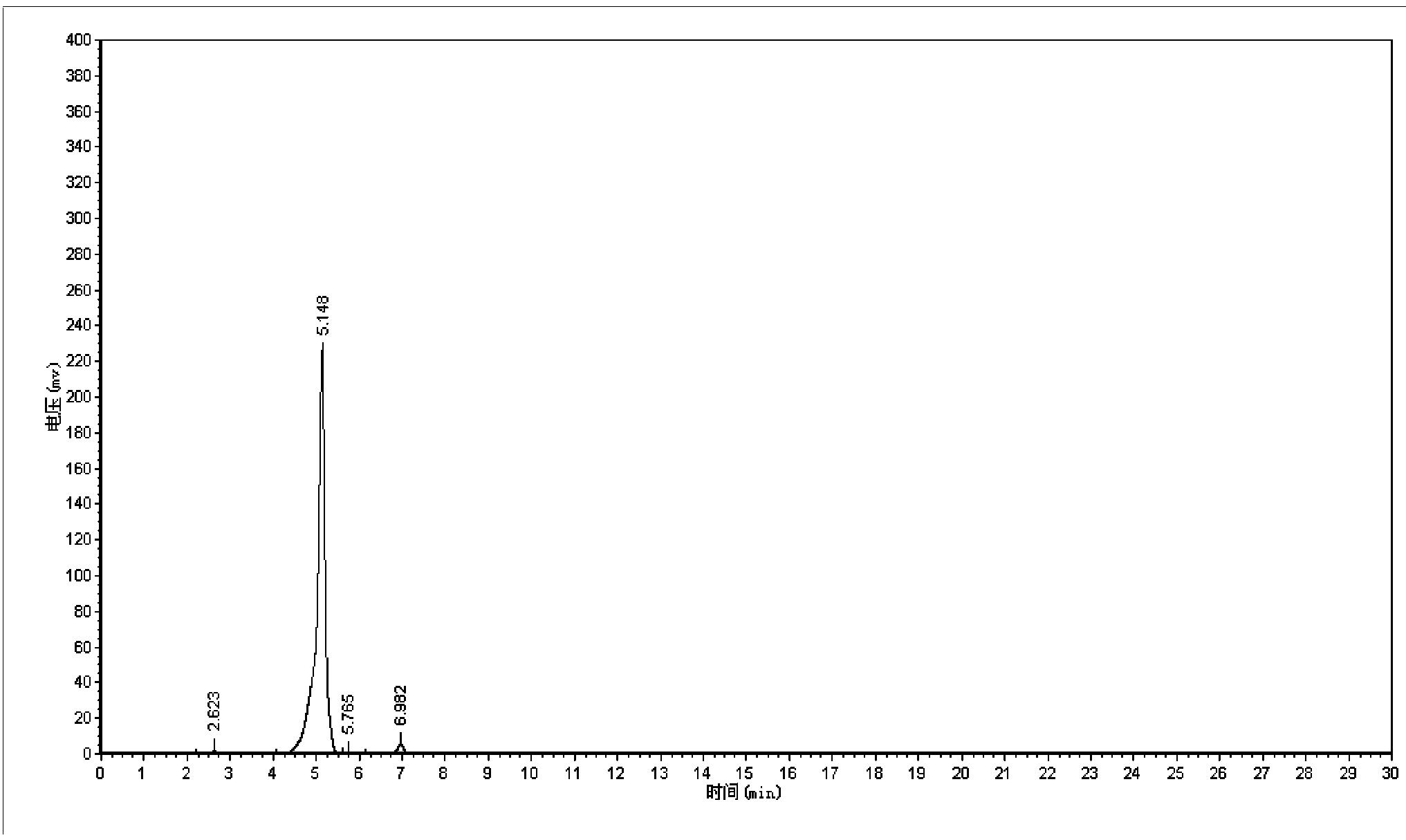
Synthetic method of 5-cholro-8-quinoline oxyacetic acid
A technology of quinolineoxyacetic acid and a synthetic method, which is applied in the field of synthesis of 5-chloro-8-quinolineoxyacetic acid, can solve the problems of environmental pollution, low yield, and corrosion of equipment in the chlorination step, so as to avoid safety problems and environmental pollution problems, high product yields, and the effects of mild production process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0020] Specific embodiment one: the synthetic method of a kind of 5-chloro-8-quinoline oxyacetic acid of the present embodiment is completed according to the following steps:
[0021] 1. Mix 8-quinolinyloxyacetic acid with 12% to 28% aqueous hydrochloric acid solution by mass to prepare an 8-quinolinyloxyacetic acid electrolyte with a concentration of 5 to 150g / L; 2. 1. Place the prepared electrolyte solution in a single-chamber electrolytic cell, use platinum sheets as cathode and anode electrodes, and perform electrolytic chlorination for 0.5-5 hours under the condition of current intensity of 0.2-0.8A; The hydrochloric acid was removed by pressure distillation to obtain the crude product of 5-chloro-8-quinolineoxyacetic acid, and then the crude product of 5-chloro-8-quinolineoxyacetic acid was refined by recrystallization method to obtain 5-chloro-8-quinoline Pure oxyacetic acid.
specific Embodiment approach 2
[0022] Embodiment 2: This embodiment differs from Embodiment 1 in that: the concentration of 8-quinolinyloxyacetic acid in step 2 is 20-120 g / L. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0023] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that the concentration of 8-quinolinyloxyacetic acid in step 2 is 50-120 g / L. Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com