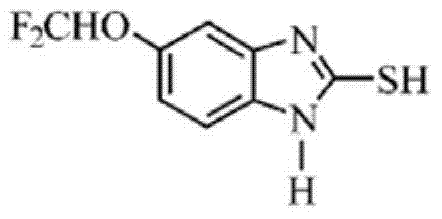
Method for synthesizing 5-difluoromethoxy-2-mercapto-1H-benzimidazole
A technology of difluoromethoxy o-phenylenediamine and difluoromethoxy, applied in the field of synthesis of 5-difluoromethoxy-2-mercapto-1H-benzimidazole, can solve the problem of single reaction temperature, organic There are many solvents and high cost, etc., to achieve the effect of simple process operation, mild reaction conditions and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] In a four-neck flask equipped with mechanical stirring, reflux condenser, and thermometer, mix lye: 6.6 grams of sodium carbonate + 20 grams of tap water, stir until completely dissolved, slightly lower the temperature, and add 4-difluoromethoxy o-phenylenediamine 34.8 g, stirred for 20 min. Raise the temperature to 30°C, add 20 grams of carbon disulfide dropwise at 25-35°C, keep warm and condense at 30-40°C for 6 hours, continue to heat up to 60-70°C and keep warm for 6 hours, after the ring closure is complete, add activated carbon for decolorization, and adjust the pH of the filtrate with sulfuric acid to 5-6, filtered, washed, and dried to obtain 33.2 grams of 5-difluoromethoxy-2-mercapto-1H-benzimidazole, based on 4-difluoromethoxy o-phenylenediamine, the yield was 95.4 %.
Embodiment 2
[0040] In a four-necked flask equipped with a mechanical stirrer, a reflux condenser, and a thermometer, mix lye: 5.5 grams of sodium hydroxide + 25 grams of tap water, stir until completely dissolved, cool down slightly, and add 4-difluoromethoxy phthalate 34.8 g of amine, stirred for 20 min. Raise the temperature to 40°C, add 28 grams of carbon disulfide dropwise at 35-45°C, keep warm and condense at 40-45°C for 3 hours after dropping, continue to heat up to 80-90°C and keep warm for 1 hour, after the ring closure is complete, add activated carbon for decolorization, and adjust the pH of the filtrate with hydrochloric acid to 5. Filter, wash, and dry to obtain 37.4 g of 5-difluoromethoxy-2-mercapto-1H-benzimidazole, with a yield of 107.5% based on 4-difluoromethoxy-o-phenylenediamine.
Embodiment 3
[0042]In a four-necked flask equipped with mechanical stirring, a reflux condenser, and a thermometer, prepare lye: 13.0 grams of potassium hydroxide + 30 grams of tap water, stir until completely dissolved, slightly lower the temperature, and add 4-difluoromethoxy phthalate 34.8 g of amine, stirred for 20 min. Raise the temperature to 40°C, add 28 grams of carbon disulfide dropwise at 35-45°C, keep warm and condense at 40-45°C for 3 hours after dropping, continue to heat up to 80-90°C and keep warm for 3 hours, after the ring closure is complete, add activated carbon for decolorization, and adjust the pH of the filtrate with phosphoric acid to 5. Filter, wash, and dry to obtain 37.8 g of 5-difluoromethoxy-2-mercapto-1H-benzimidazole, with a yield of 108.6% based on 4-difluoromethoxy-o-phenylenediamine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com