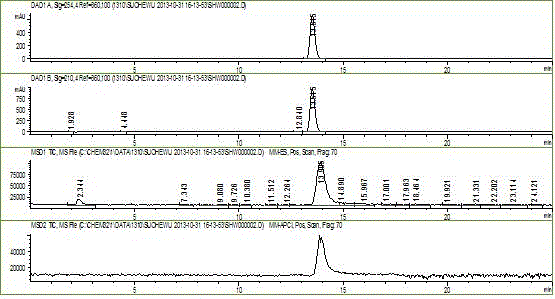
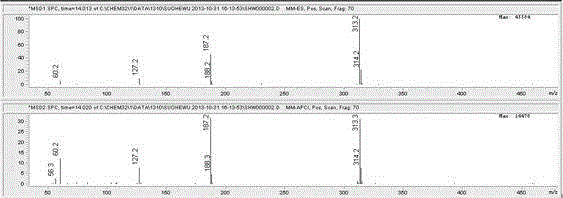
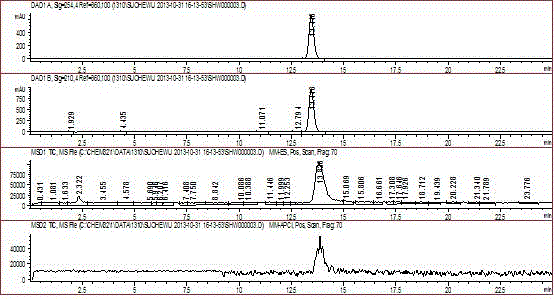
Method for preparing levonorgestrel intermediate condensation compound
A technology of levonorgestrel and condensate, which is applied in the field of Grignard reaction of levonorgestrel intermediates, can solve the problems of affecting the conversion rate and yield of the condensation reaction, unable to carry out the condensation reaction, unable to condense, etc., and achieves elimination of dehydration. The effect of hydroxyl risk, reducing the amount of recycling and ensuring quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Put 10.5Kg of magnesium chips and 1Kg of iodine into the dry reaction tank, then replace the reaction system with nitrogen, then add 20L of tetrahydrofuran into the reaction tank, stir and raise the temperature to about 30℃ to initiate, and then heat to reflux after it stabilizes. Extract 180L of tetrahydrofuran plus 32.5L of ethylene bromide and mix and pump to a high-level storage tank. Add the ethylene bromide and tetrahydrofuran solution dropwise under reflux conditions until the dripping is completed. Continue refluxing for 5 hours. After the reaction is complete, cool to below about 15°C. Add 6-methoxy-1-tetrahydrofuran solution (25Kg of 6-methoxy-1-tetrahydrofuran and 150L tetrahydrofuran), drip and react at room temperature for more than 5 hours, TLC detection, the reaction is complete At 10℃~30℃, add ammonium chloride saturated aqueous solution dropwise to terminate the reaction, stand still to separate the THF solution, extract the aqueous layer with THF, combin...
Embodiment 2
[0040] Put 10Kg of magnesium chips and 0.2Kg of iodine into the dry reaction tank, then replace the reaction system with argon, then add 10L of tetrahydrofuran to the reaction tank, stir and raise the temperature to about 20℃ to initiate, and then heat it to reflux after it stabilizes. Extract 210L of tetrahydrofuran and 32L of ethylene bromide and mix and pump to the high-level storage tank. Add the ethylene bromide and tetrahydrofuran solution dropwise under reflux conditions until the dripping is complete. Continue refluxing for 5 hours. After the reaction is complete, cool down to below about 15°C and add dropwise 6-Methoxy-1-Tetrahydrofuran solution (prepared with 25Kg of 6-Methoxy-1-Tetrahydrofuran and 160L of tetrahydrofuran), after dripping, react at room temperature for more than 5 hours, TLC detection, the reaction is complete, Add ammonium chloride saturated aqueous solution dropwise at 10℃~30℃ to stop the reaction, stand still to separate the THF solution, extract th...
Embodiment 3
[0043] Put 15Kg of magnesium chips and 0.8Kg of iodine into the dry reaction tank, then replace the reaction system with argon, then add 40L of tetrahydrofuran to the reaction tank, stir and raise the temperature to about 25℃ to initiate, and then heat it to reflux after it stabilizes. Extract 160L of tetrahydrofuran plus 35L of ethylene bromide and mix and pump to a high-level storage tank. Add the ethylene bromide and tetrahydrofuran solution dropwise under reflux conditions until the dripping is complete. Continue refluxing for 5 hours. After the reaction is complete, cool the temperature to below about 15°C and add dropwise 6-Methoxy-1-Tetrahydrofuran solution (prepared with 25Kg of 6-Methoxy-1-Tetrahydrofuran and 180L tetrahydrofuran), after dripping, react at room temperature for more than 5 hours, TLC detection, the reaction is complete, Add ammonium chloride saturated aqueous solution dropwise at 10℃~30℃ to stop the reaction, stand still to separate the THF solution, ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com