Process for synthesizing nitrocyclohexane by liquid phase nitration
A technology for nitrocyclohexane and liquid-phase nitration, which is applied in the field of acidic ionic liquid catalysts, can solve the problems of difficulty in obtaining high-purity nitrocyclohexane, complicated experimental operations, easy decomposition of catalysts, etc. High catalytic activity, the effect of promoting recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthetic steps of the quaternary ammonium salt acidic ionic liquid catalyst of one-step synthesis:
[0026] In the first step, put 5.91g (0.1mol) trimethylamine aqueous solution in a 100mL three-necked flask, and slowly add 10.0g (0.1mol) concentrated sulfuric acid (98%) dropwise in an ice-water bath (below 5°C), and the dropwise addition is completed Then continue to stir for 1h.
[0027] In the second step, the temperature of the reaction solution in the first step was raised to 80° C. and stirred for 4 hours.
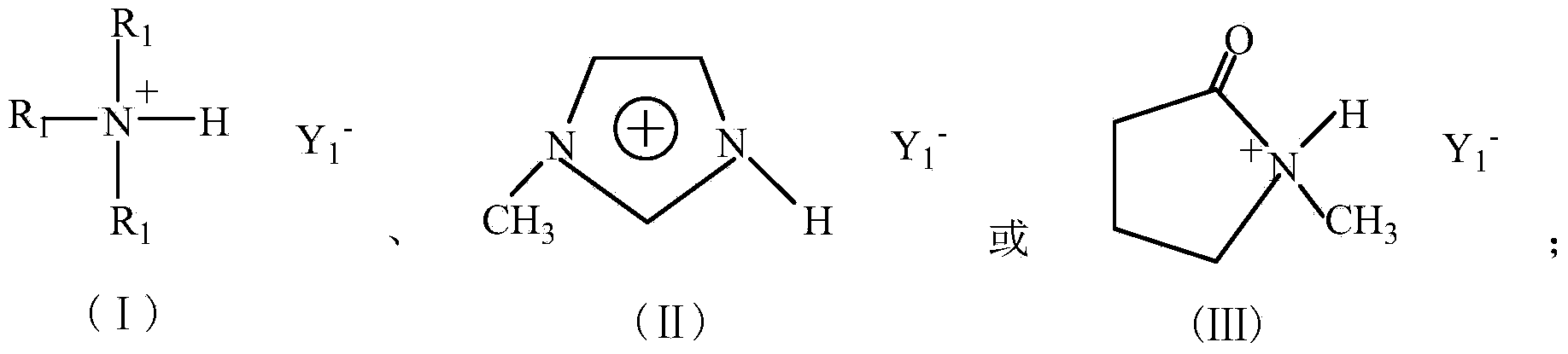
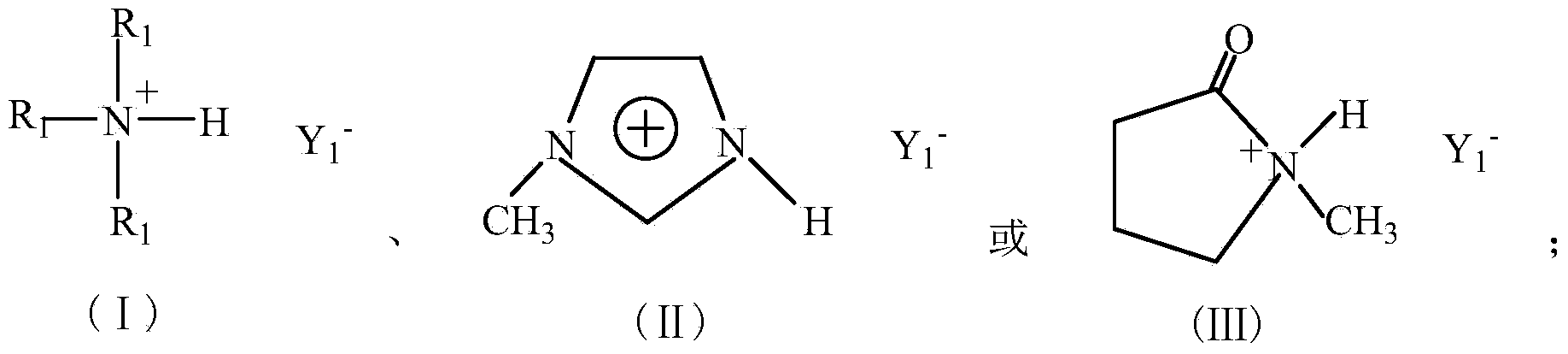
[0028] In the third step, the crude product obtained in the second step is cooled to room temperature, washed three times with ethyl acetate, and the ethyl acetate and the like are removed by rotary evaporation under reduced pressure, and then vacuum-dried at 80°C for 12 hours to obtain a colorless, transparent and viscous Ionic liquid a: trimethylamine bisulfate ionic liquid ([(CH 3 ) 3 N]HSO 4 ). See the compound (I) for the structural formula, R ...
Embodiment 2
[0030] Step is with embodiment 1, difference is, in the first step, add 10.1g (0.1mol) triethylamine aqueous solution, the 3rd step obtains yellow viscous solid b: triethylamine bisulfate ionic liquid ([(CH 3 CH 2 ) 3 N]HSO 4 ). See the compound (I) for the structural formula, R 1 =C 2 h 5 , Y 1 =HSO 4 .
Embodiment 3
[0032] Step is the same as Example 1, and the difference is that in the first step, 8.21g (0.1mol) N-methylimidazole is added, and the third step obtains yellow transparent viscous liquid c: N-methylimidazole bisulfate ionic liquid ([ Hmim] HSO 4 ). See compound (Ⅱ) for structural formula, Y 1 =HSO 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com