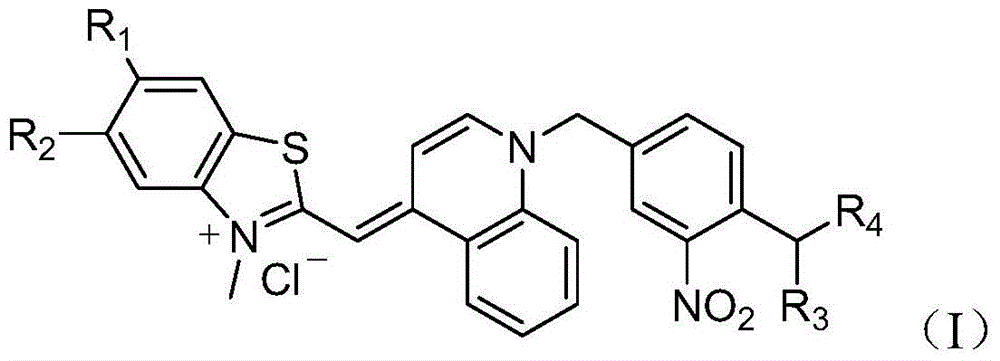
A kind of asymmetric cyanine dye compound and its application
A technology for compounds and cyanine dyes, which can be used in organic dyes, methine/polymethine dyes, analytical materials, etc., and can solve problems such as insufficient quenching efficacy and limitation of signal kinetic characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the synthesis of compound (II)
[0043]
[0044] 4-Methylquinoline (4.8ml, 0.0363mol) with methyl 2-(4-(bromomethyl)-2-nitrophenyl)propionate (according to Naoki Yamakawa et al., Bioorganic & Medicinal Chemistry 19 (2011) 3299 -3311 preparation) (16.44g, 0.05445mol) were mixed, reacted at 110°C overnight, after cooling, added 20ml of methanol to dissolve, suspended and steamed to obtain a viscous solid, then added 200ml of acetone and stirred, after stirring for 2 hours, the product precipitated, filtered , dried to obtain product 6.4g.
[0045] Product NMR data 1 H NMR: 1.51 (s, 3H), 2.66 (s, 3H), 3.6 (d, 3H), 3.7 (m, 1H), 5.95 (s, 2H), 7.5-8.6 (m, 9H).
Embodiment 2
[0046] Embodiment 2: Synthesis of compound (I-1)
[0047]
[0048] 3-Methyl-2-methylthiobenzothiazole p-toluenesulfonate (5g, 0.014mol) and compound (II) (6.33g, 0.01mol) were dissolved in 20ml DMF, and 3.9ml of Triethylamine, then stirred overnight, after the reaction, pour the product into water, add 5% hydrochloric acid dropwise, filter the product after precipitation, dissolve the filtered product in 30ml of 1:1 methanol and water, add 5.6g of hydrogen Sodium oxide was hydrolyzed. After hydrolysis, concentrated hydrochloric acid was added dropwise again. After the product was precipitated, it was filtered and dried to obtain the final product.
[0049] Product NMR data 1 H NMR: 1.46 (s, 3H), 3.7 (m, 1H), 4.39 (s, 3H), 5.95 (s, 2H), 6.77 (s, 1H), 7.5-8.6 (m, 13H).
Embodiment 3
[0050] Embodiment 3: the synthesis of compound (I-2)
[0051]
[0052] Compound (I-1) (30mg, 0.056mmol) was dissolved in 0.5ml DMF, 0.05ml DIEA was added and TSTU (34mg, 0.12mmol) was added. The mixture was stirred at room temperature for 2-3 hours, and the reaction was monitored by TLC. After the reaction, 2ml of 5% HCl was added, and the precipitate was washed with dilute hydrochloric acid, filtered, and dried to obtain 30 mg of the product.
[0053] NMR data 1 H NMR: 1.46 (s, 3H), 2.64 (m, 4H), 3.7 (m, 1H), 4.39 (s, 3H), 5.95 (s, 2H), 6.77 (s, 1H), 7.5-8.6 (m , 13H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com