Method for measuring content of magnesium element in aluminum alloy
A measurement method and aluminum alloy technology, which is applied in the direction of material analysis by observing the influence of chemical indicators, and analysis by making materials undergo chemical reactions, can solve the problems of poor complexation ability, poor masking effect, magnesium content, etc. Inaccurate and other problems, to achieve the effect of accurate measurement results, environmentally friendly reagents, and obvious end points
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Add 2g cast aluminum alloy ZL6 to 30mL1mol / LNaOH solution, add 5mL10wt%H 2 o 2 , react at 50°C for 40 minutes, filter, and wash the filter cake with water;
[0024] (2) Dissolve the filter cake in 20mL of 0.2mol / L hydrochloric acid solution, add 10mL of combined masking agent, use ammonia buffer to adjust the pH of the solution to 9.5, and add 0.02g of chrome black T;
[0025] (3) Titrate the solution obtained in step (2) with EDTA until the solution changes from red to blue, which is the end point.
[0026] (4) According to the formula Calculate the mass percentage of magnesium in the aluminum alloy,
[0027] In the formula: C—EDTA standard titration solution concentration, mol / L;
[0028] V—the volume of EDTA standard titration solution consumed by titration, L;
[0029] m 0 - the amount of sample to be weighed, g;
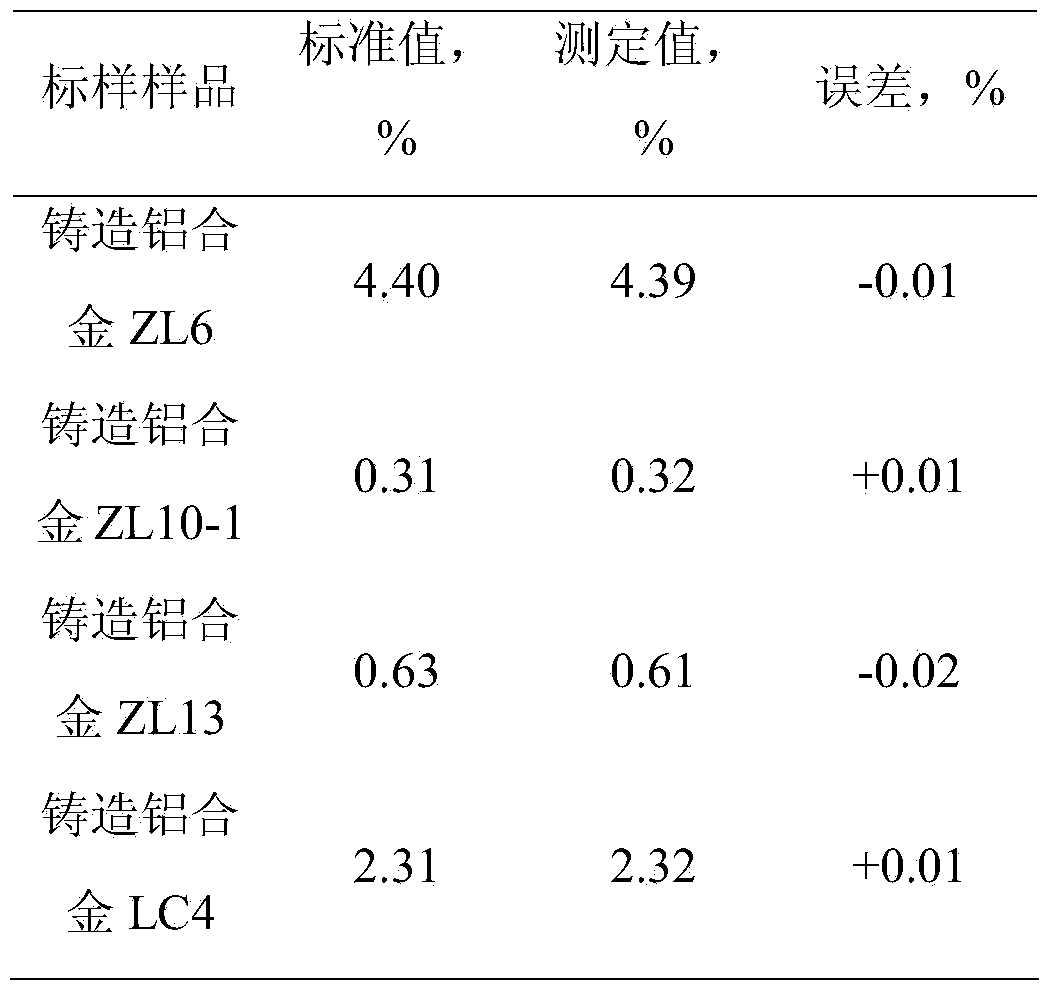
[0030] The results are shown in Table 1.
Embodiment 2
[0032] (1) Add 2g cast aluminum alloy ZL10-1 to 30mL1mol / L NaOH solution, add 5mL10wt%H 2 o 2 , react at 50°C for 2h, filter, and wash the filter cake with water;
[0033] (2) Dissolve the filter cake in 20mL of 0.2mol / L hydrochloric acid solution, add 10mL of combined masking agent, use ammonia buffer to adjust pH=9.8, and add 0.02g of chrome black T;
[0034] (3) Titrate the solution obtained in step (2) with EDTA until the solution changes from red to blue;
[0035] (4) According to the formula Calculate the mass fraction of magnesium in the aluminum alloy,
[0036] In the formula: C—EDTA standard titration solution concentration, mol / L;
[0037] V—the volume of EDTA standard titration solution consumed by titration, L;
[0038] m 0 - the amount of sample to be weighed, g;
[0039] The results are shown in Table 1.
Embodiment 3
[0041] (1) Add 2g cast aluminum alloy ZL13 to 30mL1mol / L NaOH solution, add 5mL10wt%H 2 o 2 , react at 50°C for 3h, filter, and wash the filter cake with water;
[0042] (2) Dissolve the filter cake in 20mL of 0.2mol / L hydrochloric acid solution, add 10mL of combined masking agent, use ammonia buffer to adjust the pH of the solution to 9.8, and add 0.02g of chrome black T;
[0043] (3) Titrate the solution obtained in step (2) with EDTA until the solution changes from red to blue;
[0044] (4) According to the formula Calculate the mass fraction of magnesium in the aluminum alloy,
[0045] In the formula: C—EDTA standard titration solution concentration, mol / L;
[0046] V—the volume of EDTA standard titration solution consumed by titration, L;
[0047] m 0 - the amount of sample to be weighed, g;
[0048] The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com