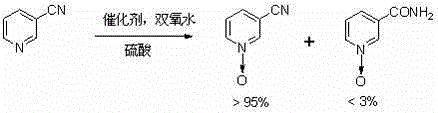
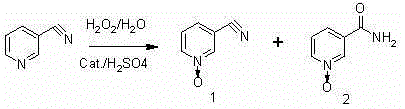
A kind of preparation method of 3-cyano-pyridine n-oxide
A technology of cyanopyridine and oxide, which is applied in the field of preparation of 3-cyano-pyridine N-oxide, can solve the problems of being unsuitable for industrialization and expensive catalyst, and achieves low price, improved purity and yield, and improved The effect of synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 100 mL of water, 7.5 g of concentrated sulfuric acid, 5 g of silicomolybdic acid and 700 g of 3-cyanopyridine were put into the reaction kettle in turn. Heating, slowly raising the temperature to 75-85°C and keeping it, and evenly adding 750mL of 30% hydrogen peroxide dropwise within 10 hours, after dropping, keep warm for 8 hours. Then cool down to below 15°C, centrifuge, and dry to obtain 768 grams of 3-cyanopyridine-N-oxide, with a yield of 95.1%, a purity of 96.3% (HPLC area normalization method), and a melting point of 169-171°C. The value is 172°C (Russian Journal of General Chemistry, 1993, 63, 1605; the same applies to the following examples).
Embodiment 2
[0016] 200 mL of water, 15 g of concentrated sulfuric acid, 10 g of phosphomolybdic acid and 1400 g of 3-cyanopyridine were put into the reaction kettle in turn. Heat, slowly raise the temperature to 86-94°C and keep it, and evenly add 1550mL of 30% hydrogen peroxide dropwise within 8 hours, and keep warm for 6 hours after the drop is completed. Then lower the temperature to below 15°C, centrifuge, and dry to obtain 1550 g of 3-cyanopyridine nitrogen oxide, with a yield of 96.1%, a purity of 95.3% (HPLC area normalization method), and a melting point of 168-171°C.
Embodiment 3
[0018] Put 100mL of water, 7.5g of concentrated sulfuric acid, 6.0g of phosphotungstic acid and 700g of 3-cyanopyridine into the reaction kettle in turn. Heat, slowly raise the temperature to 90-95°C, add 750mL of 30% hydrogen peroxide dropwise evenly within 10 hours, and keep warm for 8 hours after dropping. Then lower the temperature to below 15°C, centrifuge, and dry to obtain 765 g of 3-cyanopyridine-N-oxide, with a yield of 94.7%, a purity of 96.8% (HPLC area normalization method), and a melting point of 168-172°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com