2-quinolinone compound and preparation method and application thereof
A technology for quinolinones and compounds, which is applied in the application of detecting Hg2+, in the field of preparation of 2-quinolinone compounds, can solve the problems of complicated operation, cumbersome sensor preparation method, etc., and achieves simple detection process, convenient preparation, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
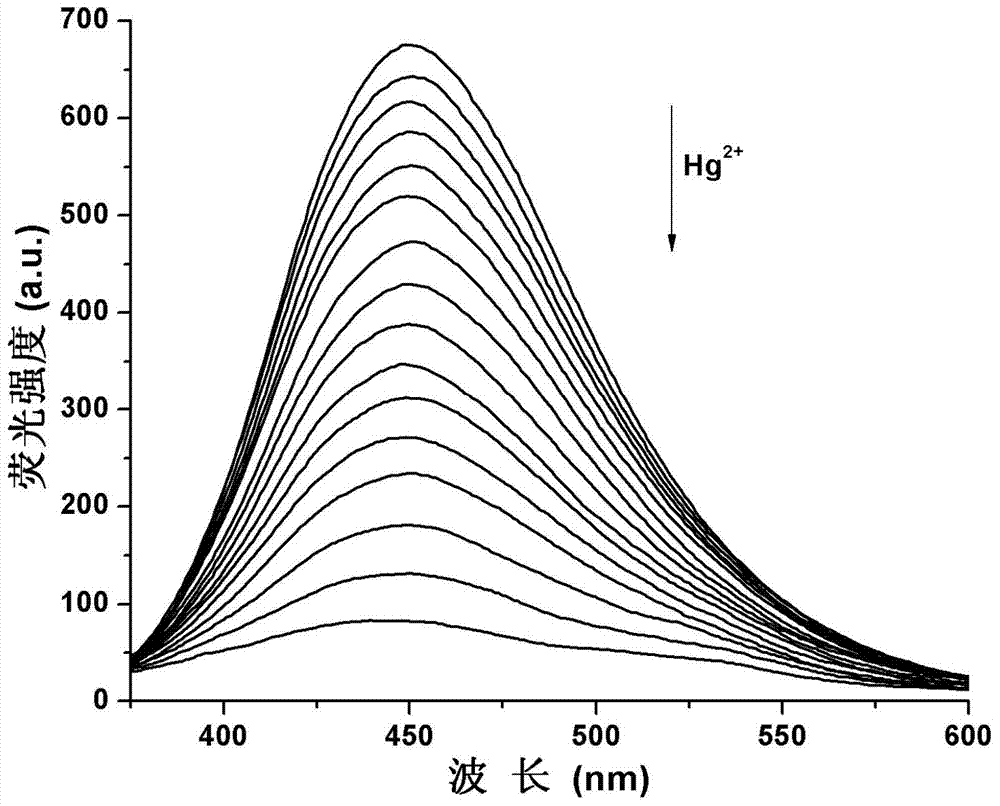
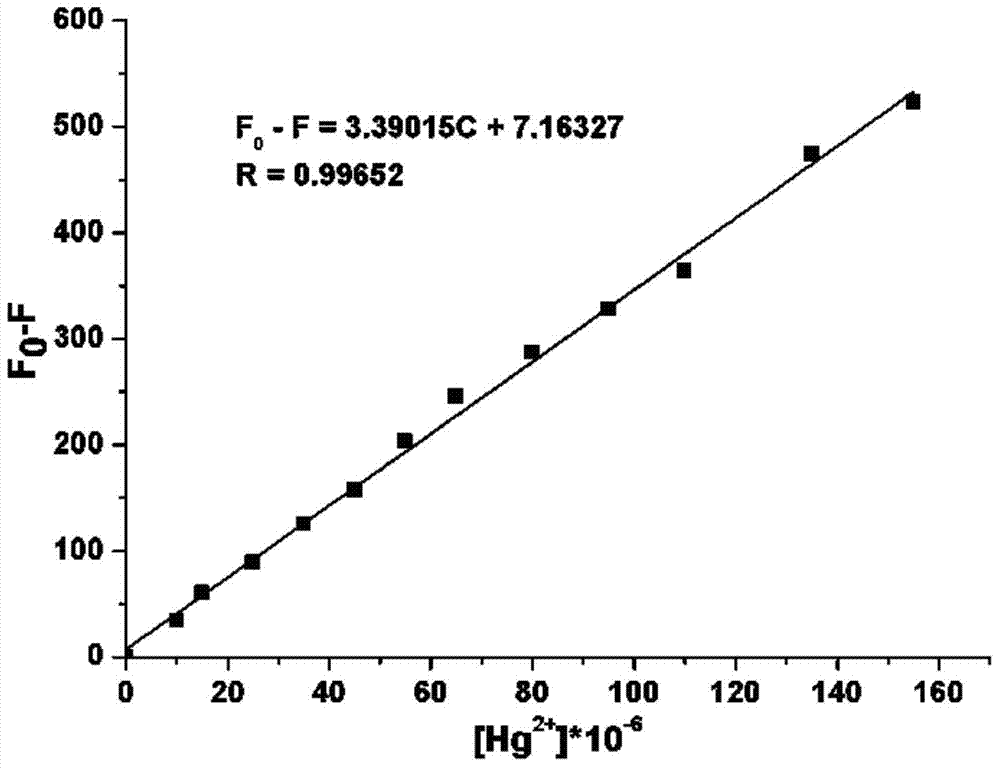
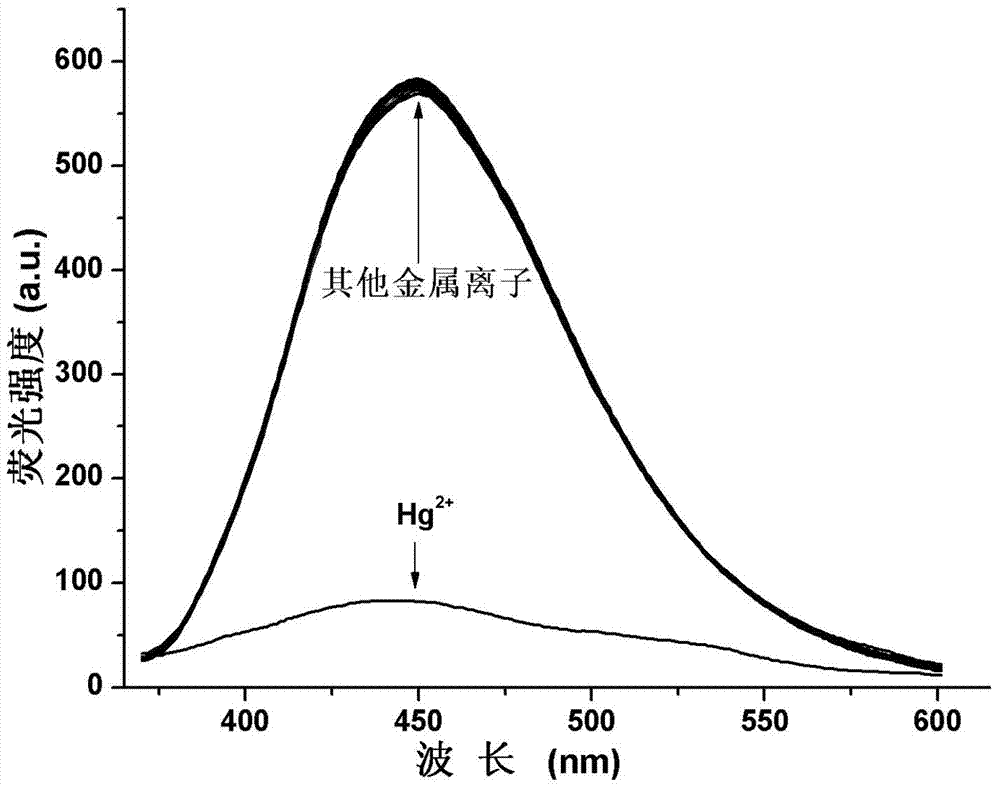
Image
Examples
Embodiment 1
[0030] Preparation of 3-(2-benzimidazolyl)-6,7-difluoro-2-quinolinone (BIDFQ)
[0031] (1) In a 250mL reaction flask, add 38.7mL (0.5mol) DMF, cool in an ice-water bath to 0°C, add 116.3mL (1.25mol) phosphorus oxychloride dropwise under stirring, and control the dropping rate to keep the temperature of the reaction system At 0±2°C, continue the reaction at low temperature for 0.5 hours after the addition is complete, then add 42.75g (0.25mol) of 3,4-difluoroacetanilide, heat to 70°C and keep the reaction for 16 hours. After cooling, the reaction solution was poured into crushed ice to precipitate a yellow solid, which was filtered, washed with water, and dried to obtain 9 g of 2-chloro-6,7-difluoro-3-quinolinecarbaldehyde, with a yield of 15.8%.
[0032] (2) In a 50mL reaction flask, add 25mL of 70% acetic acid aqueous solution and 2.275g (10mmol) of 2-chloro-6,7-difluoro-3-quinolinecarbaldehyde, and heat to reflux for 6 hours. Cool, filter, wash with water, and dry to obtain...
Embodiment 2
[0040] Preparation of 3-(2-benzimidazolyl)-6,7-difluoro-2-quinolinone (BIDFQ)
[0041] (1) The preparation of 2-chloro-6,7-difluoro-3-quinolinecarbaldehyde is the same as in Example 1;
[0042] (2) The preparation of 3-formyl-6,7-difluoro-2-quinolinone is the same as in Example 1;
[0043] (3) In a 10mL reaction flask, add 5mL absolute ethanol and 209mg (1mmol) 3-formyl-6,7-difluoro-2-quinolinone, start stirring, then add 108mg (1mmol) phthalate Amine and 17.2 mg p-toluenesulfonic acid, and then heated to reflux for 7 hours. Cool, filter, and recrystallize with acetone to obtain 184 mg of 3-(2-benzimidazole)-6,7-difluoro-2-quinolinone, with a yield of 62%.
Embodiment 3
[0045] Preparation of 3-(2-benzimidazolyl)-6,7-difluoro-2-quinolinone (BIDFQ)
[0046] (1) The preparation of 2-chloro-6,7-difluoro-3-quinolinecarbaldehyde is the same as in Example 1;
[0047] (2) The preparation of 3-formyl-6,7-difluoro-2-quinolinone is the same as in Example 1;
[0048] (3) In a 10mL reaction flask, add 5mL absolute ethanol and 209mg (1mmol) 3-formyl-6,7-difluoro-2-quinolinone, start stirring, then add 108mg (1mmol) phthalate Amine and 18.6 mg of boric acid were then heated to reflux for 10 hours. Cool, filter, and recrystallize with acetone to obtain 166 mg of 3-(2-benzimidazole)-6,7-difluoro-2-quinolinone, with a yield of 56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com