Wheat cellulose particles and preparation method thereof
A technology of wheat cellulose and hypromellose, which is applied in the field of medical care, can solve the problems of high dosage and low dietary fiber content, and achieve the effects of less consumption, promotion of proliferation, and intestinal laxation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of Wheat Cellulose Granules
[0024] Prescription: 2730g of wheat cellulose, 270g of xylitol, 95% ethanol solution of 4% hypromellose in right amount.
[0025] Preparation method: Mix the wheat cellulose and xylitol in the prescribed amount evenly, use an appropriate amount of 4% hypromellose in 95% ethanol as a binder, wet granulate, dry below 80°C, granulate, pack, Instantly.
[0026] The process verification results are shown in Table 2:
[0027]
[0028] Three batches of product inspection results
[0029] Three batches of products were self-inspected, and the total number of microbiological index bacteria were 60, 50, and 60Cfu / g, coliforms <30MPN / 100g, mold and yeast <10Cfu / g, and other pathogenic bacteria were not detected. Efficacy The contents of insoluble dietary fiber in ingredients or iconic ingredients are 79.5g / 100g, 79.3g / 100g, and 79.1g / 100g respectively, and other physical and chemical indicators all meet the requirements o...
Embodiment 2
[0031] Example 2: Animal Safety Toxicology Test of Wheat Cellulose Granules
[0032] The present invention is subjected to acute toxicity test, Ames test, mouse bone marrow polychromatic erythrocyte micronucleus test, mouse sperm deformity test, and 30-day feeding test by Shandong University Health Analysis and Testing Center.
[0033] In the acute toxicity test, the MTD of the wheat cellulose granules of the present invention is greater than 18.0 g / kg.bw, and according to the acute toxicity classification, the wheat cellulose granules of the present invention are non-toxic.
[0034] In the genetic toxicity test, the results of Ames test, mouse bone marrow polychromatic erythrocyte micronucleus test and mouse sperm abnormality test were all negative.
[0035] In the 30-day feeding test, rats were fed with feeds prepared from wheat cellulose granules of the present invention that were 25 times, 50 times, and 100 times the daily recommended amount of the human body. For 30 conse...
Embodiment 3
[0037] Embodiment 3: Wheat cellulose granule laxative function animal experiment report
[0038] 1 Materials and methods
[0039] 1.1 Sample: The wheat cellulose granules of the present invention are provided by Jinan Hailijian Biotechnology Co., Ltd. Its properties are gray particles.
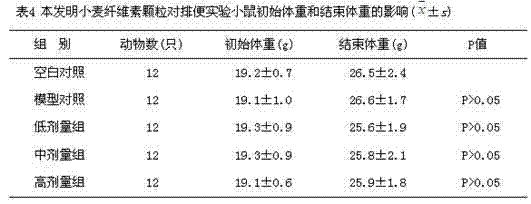
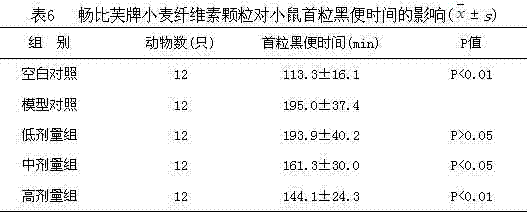
[0040] Experimental animals: Healthy SPF Kunming male mice provided by the Experimental Animal Center of Shandong University were selected, and the experimental animal production license number is SCXK (Lu) 20120004. The experimental environment temperature is 22°C±2°C, and the relative humidity is 50%±10%. The certificate number of the experimental animal environment facility is Ludonghuanzi No. H2002100112. Weight 18-22g. According to the weight level of the mice, they were randomly divided into 10 groups, with 12 mice in each group, 60 of which were subjected to the small intestine motility test, and the other 60 were subjected to the measurement experiment of the time of the fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com