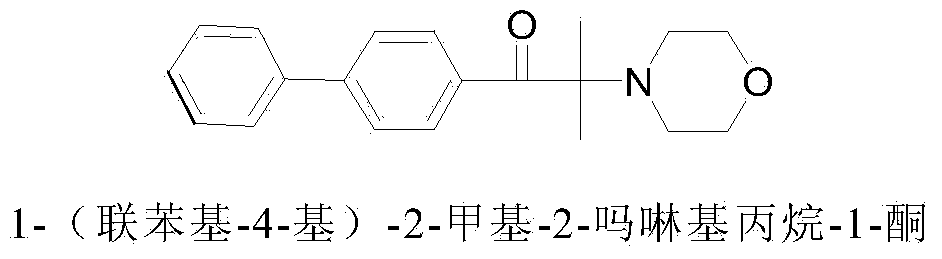
Synthetic method of 1-(biphenyl-4-yl)-2-methyl-2-morpholinopropan-1-one
A technology of morpholino propane and a synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of excessive waste water, difficult industrial implementation and high cost, and achieve the effects of simplifying the process, reducing the production cycle and fast reaction speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Synthesis of 1-(biphenyl-4-yl)-2-methyl-2-morpholinopropane-1-one
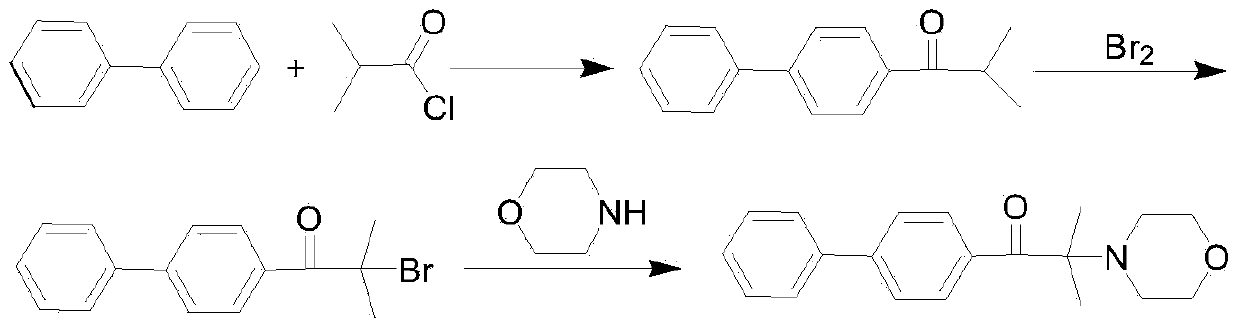
[0037] 1) Add 450 grams of isobutyric acid into a 1000 ml reaction kettle, raise the temperature to 45°C, then add 320 grams of phosphorus trichloride, keep it at 55°C for 6 hours, follow the reaction by GC, and stop the reaction after the disappearance of the raw material isobutyric acid. The inorganic acid in the lower layer was separated to obtain 541.8 grams of isobutyryl chloride, the GC content was 99.4%, and the product yield was about 99.6%.
[0038] 2) Add 376 grams of anhydrous dichloroethane to a 1000 ml reaction bottle, cool down to 10°C, add 90 grams of anhydrous aluminum trichloride and 100 g of biphenyl, continue to cool down to 5°C, and add the prepared in step 1) Acyl chloride 76 grams, kept at 5°C for 5 hours, GC followed the reaction, after the raw material biphenyl disappeared, the reaction was terminated. Pour the above reaction solution into 240 g of 3% dilute hydrochlo...
Embodiment 2
[0041] Example 2: Synthesis of 1-(biphenyl-4-yl)-2-methyl-2-morpholinopropane-1-one
[0042] 1) Add 376 g of anhydrous chlorobenzene into a 1000 ml reaction bottle, cool down to 10°C, add 90 g of anhydrous aluminum trichloride and 100 g of biphenyl, continue cooling down to 5°C, add the acid chloride 76 prepared in Example 1 gram, kept at 5° C. for 5 hours, and followed the reaction by GC. After the raw material biphenyl disappeared, the reaction was terminated. The above reaction solution was poured into 240 g of 3% dilute hydrochloric acid, stirred for 1 hour, and separated to obtain a 2-methyl-1-biphenyl-1-acetone solution with a GC content of 99.5%.
[0043] 2) Add the 1-(biphenyl-4-yl)-2-methylpropan-1-one solution prepared in step 1) into a 250ml reaction bottle, raise the temperature to 90°C, then inject chlorine gas, and follow the reaction by GC , After 1-(biphenyl-4-yl)-2-methylpropan-1-one disappears, the reaction is terminated. Lower the temperature and add 133 m...
Embodiment 3
[0045] Example 3: Synthesis of 1-(biphenyl-4-yl)-2-methyl-2-morpholinopropane-1-one
[0046] 1) Add 376 grams of anhydrous dichloroethane into a 1000 ml reaction bottle, cool down to 8°C, add 95 grams of anhydrous aluminum trichloride and 100 g of biphenyl, continue to cool down to 3°C, and add the 76 grams of acid chloride were incubated at 14° C. for 4 hours, and the reaction was tracked by GC. After the raw material biphenyl disappeared, the reaction was terminated. Pour the above reaction solution into 220 grams of 3.5% dilute hydrochloric acid, stir for 1 hour, separate the liquids, the organic phase is 1-(biphenyl-4-yl)-2-methylpropan-1-one solution, GC The content is 99.0%.
[0047] 2) Add the 1-(biphenyl-4-yl)-2-methylpropan-1-one solution prepared in 1) into a 250 ml reaction bottle, raise the temperature to 90°C, then inject chlorine gas, and follow the reaction by GC , after 1-(biphenyl-4-yl)-2-methylpropan-1-one disappears, the reaction is terminated. Lower the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com