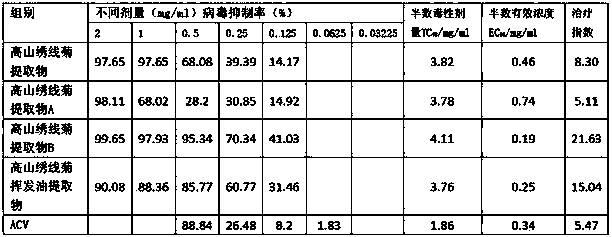
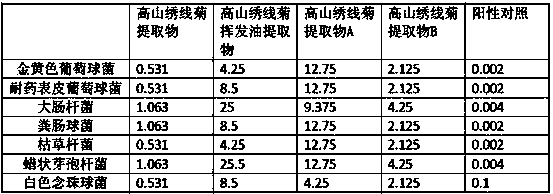
Application of spiraea alpine extract in antiviral drug preparation
A technology of Alpine meadowsweet and antiviral drug, applied in the field of biomedicine, can solve the problems such as no reports on research on antibacterial and antiviral effects of Alpine meadowsweet, and achieve the effects of preventing virus invasion and protecting cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of alpine Spiraea extract
[0036] (1) Spiraea alpine 18kg, extracted with 95% ethanol, filtered to obtain extract A and dregs A, extract A was concentrated to an alcohol-free paste, added distilled water, steam distilled, and collected the distillate , adding 4% of distillate weight β-cyclodextrin clathrate, cold storage, filtration, low-temperature drying, to obtain the clathrate of the volatile oil of Spiraea alpine;
[0037](2) Use the dregs A obtained in step (1) with 60% ethanol solution as a solvent, the extraction temperature is 80°C, the number of extractions is 2 times, the extraction time is 2 hours each time, and the amount of solvent used each time is Meadowsweet alpine 12 times the weight; filter to obtain extract B and drug residue B; extract B, recover ethanol, concentrate to relative density d=1.13, filter to obtain drug C;
[0038] (3) Pass the drug solution C obtained in step (2) through ADS-3 macroporous adsorption resi...
Embodiment 2
[0041] Embodiment 2: the preparation of alpine Spiraea extract
[0042] (1) 15kg of Spiraea alpine, extract with 95% ethanol, filter to obtain extract A and dregs A, extract A is concentrated to an alcohol-free paste, add distilled water, carry out steam distillation, and collect the distillate , adding 4% of distillate weight β-cyclodextrin clathrate, cold storage, filtration, low-temperature drying, to obtain the clathrate of the volatile oil of Spiraea alpine;
[0043] (2) Use the dregs A obtained in step (1) with 30% ethanol solution as a solvent, the extraction temperature is 95°C, the number of extractions is 1 time, and the extraction time is 4 hours each time, and the amount of solvent used each time is Spiraea alpine 15 times the weight; filter to obtain extract B and drug residue B; extract B, recover ethanol, concentrate to relative density d=1.18, filter to obtain drug C;
[0044] (3) Pass the medicinal liquid C obtained in step (2) through HP20 macroporous adsorp...
Embodiment 3
[0047] Example 3: Preparation of Spiraea Alpine Extract and Monomer Compounds
[0048] (1) 20kg of Spiraea alpine, extract with 95% ethanol, filter to obtain extract A and dregs A, concentrate extract A to a clear paste without alcohol, add distilled water, carry out steam distillation, and collect the distillate , adding 4% of distillate weight β-cyclodextrin clathrate, cold storage, filtration, low-temperature drying, to obtain the clathrate of the volatile oil of Spiraea alpine;
[0049] (2) Use the dregs A obtained in step (1) with 75% ethanol solution as a solvent, the extraction temperature is 50°C, the number of extractions is 3 times, and the extraction time is 1 hour each time, and the amount of solvent used each time is Meadowsweet alpine 6 times the weight; filter to obtain extract B and medicinal residue B; extract B, recover ethanol, concentrate to relative density d=1.10, filter to obtain medicinal liquid C;
[0050] (3) Pass the drug solution C obtained in step...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com