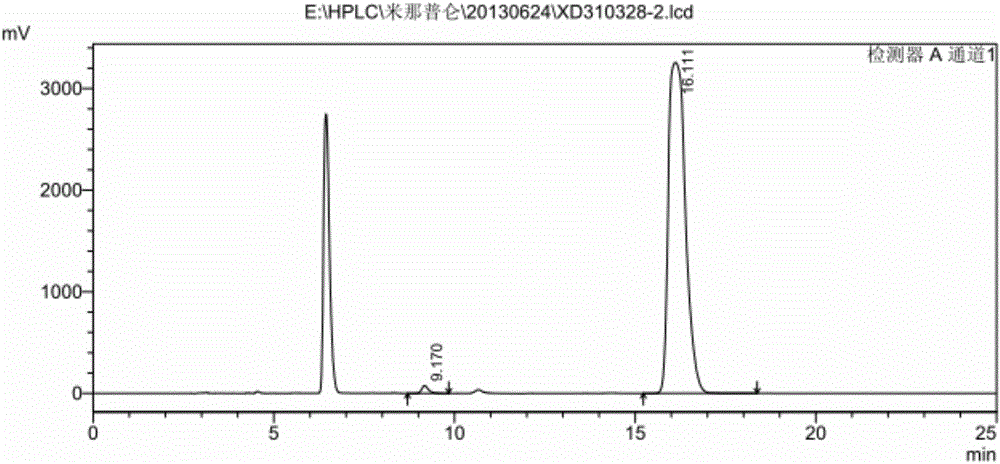
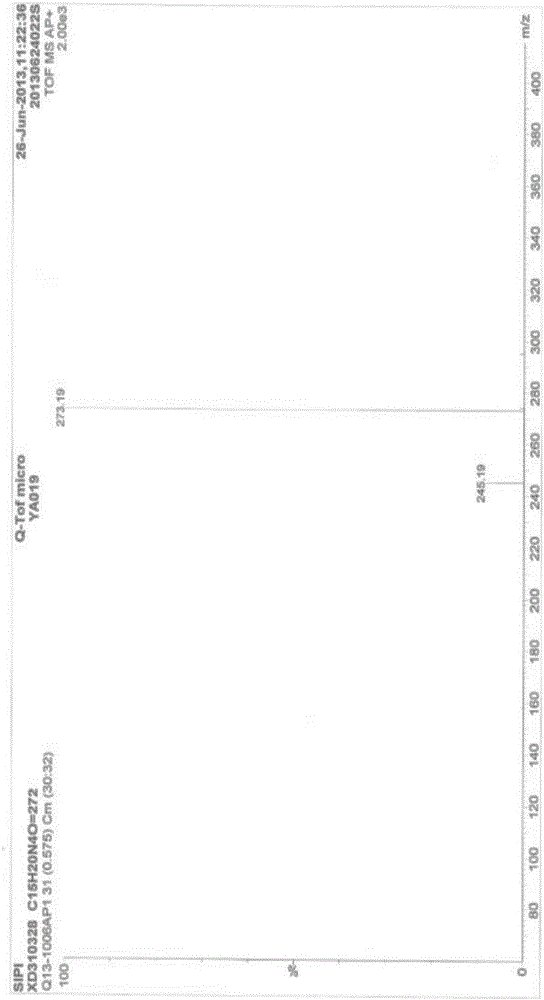
Milnacipran hydrochloride intermediate and its preparation method and application
A compound and inert solvent technology, applied in the field of synthesis of milnacipran hydrochloride, can solve problems such as being not very economical, affecting yield, etc., and achieve the effects of mild conditions, simple operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0048] Embodiment 1-3: the synthesis of formula II compound
[0049] (A) Dissolve 100 g of the compound of formula I in 500 ml of methanol, and then slowly add 137 g of thionyl chloride dropwise. After the addition is complete, react at room temperature for 10 hours. After TLC monitors that the reaction is complete, filter and filter the cake at 40 ° C. Press dry. 122 g of the product was obtained with a yield of 94.5%.
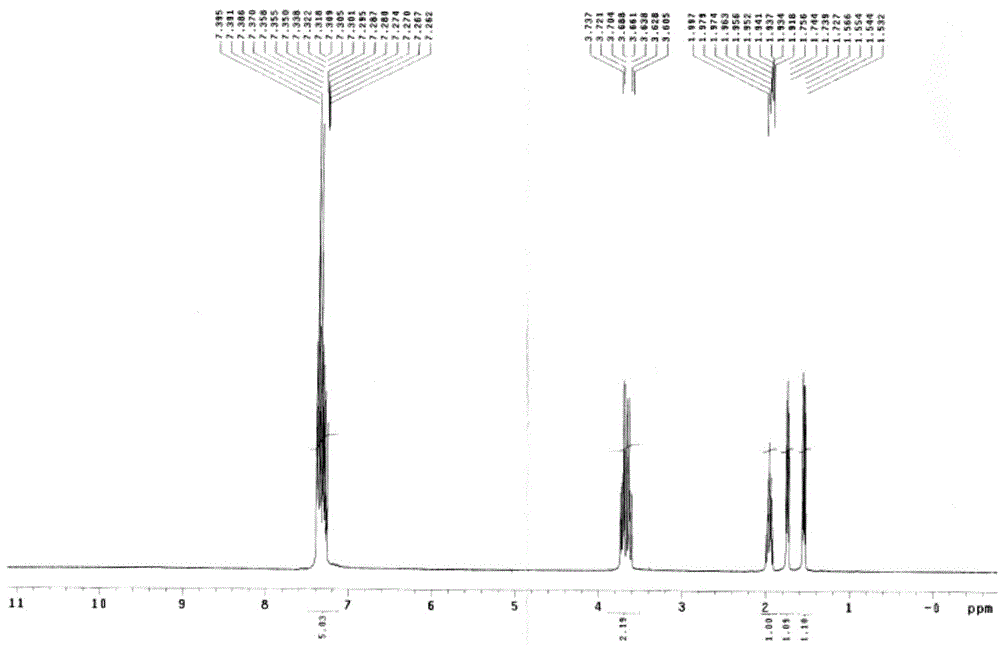
[0050] 1 HNMR (400MHz, CDCl 3 ):δ7.374-7.277(m,5H),3.685(s,3H),3.645-3.589(m,2H),1.891-1.875(m,1H),1.780-1.760(m,1H),1.493-1.467 (m,1H)
[0051] (B) Dissolve 100g of the compound of formula I in 500ml of methanol, then slowly add 137g of thionyl chloride dropwise, after the dropwise addition, react at -5-5°C for 15 hours, monitor the reaction by TLC, filter, and filter the cake in Dry under reduced pressure at 40°C. 120 g of the product was obtained with a yield of 93%.
[0052] (C) Dissolve 100 g of the compound of formula I in 500 ml of methanol, the...
Embodiment 4-5
[0053] Embodiment 4-5: the synthesis of formula III compound
[0054] (A) 100 g of compound II and 30 g of sodium azide were added to 500 ml of toluene, and reacted at 80° C. for 12 hours. After the completion of the reaction was monitored by TLC, it was cooled to room temperature, 200 ml of water was added, the liquid was extracted and separated, and the organic layer was evaporated to dryness to obtain a white solid. Filter and dry the filter cake under reduced pressure at 40°C. 91 g of the product was obtained with a yield of 88%.
[0055] (B) 100 g of compound II and 30 g of sodium azide were added to 200 ml of dimethylformamide, and reacted at 80° C. for 7 hours. After the completion of the reaction as monitored by TLC, it was cooled to room temperature, poured into 200 ml of ice water, and a solid was precipitated. Filter and dry the filter cake under reduced pressure at 40°C. 100 g of the product was obtained with a yield of 98%.
[0056] 1 HNMR (400MHz, CDCl 3 )...
Embodiment 6
[0057] Embodiment 6: the synthesis of formula IV compound
[0058] Add 100 g of the compound of formula III into the mixed solution (400 ml of toluene, 50 ml of methanol, 105 ml of 20% NaOH), and react at 80° C. for 6 hours. After the completion of the reaction was monitored by TLC, it was cooled to room temperature, and the liquid was separated. The aqueous layer was adjusted to weak acidity with 6N hydrochloric acid, and a solid was precipitated. Filter and dry the filter cake under reduced pressure at 40°C. 90 g of the product was obtained with a yield of 95.6%. (melting point: 95.1-95.7°C) 1 HNMR (400MHz, CDCl3 ):δ7.395-7.262(m,5H),3.737-3.605(m,2H),1.997-1.918(m,1H),1.756-1.727(m,1H),1.566-1.532(m,1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com