Preparation method suitable for industrial production of empagliflozin
A technology of empagliflozin and compounds, applied in the design of organic synthesis routes and in the fields of medicine and chemical industry, to achieve the effects of reducing production costs, simplifying process steps, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
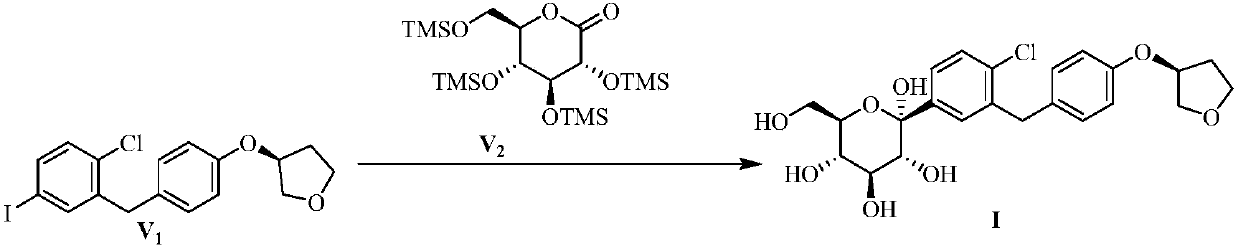
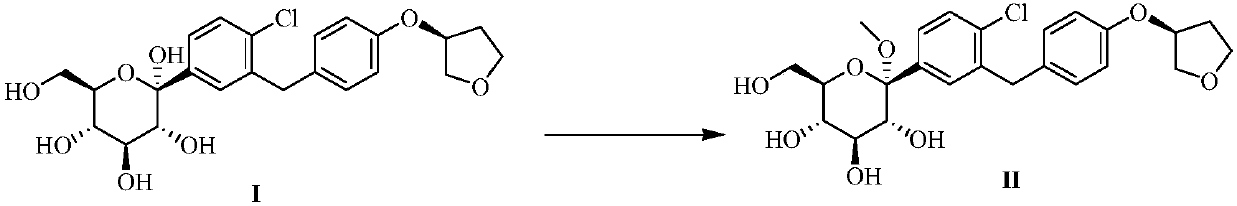
Image
Examples
Embodiment 1
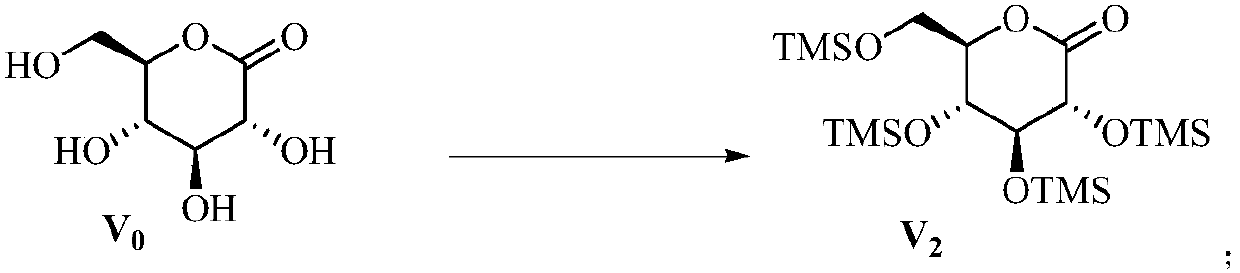
[0041] Compound V 2 Preparation of:
[0042]
[0043] Under nitrogen protection, compound V 0 (10kg, 56mol) and 50L of pyridine were added into 80L of dichloromethane, cooled to -20°C, and stirred for 10 minutes to dissolve. Slowly add trimethylchlorosilane (50 L, 168 mol) dropwise, keep the internal temperature not exceeding 10°C, return to room temperature and stir for 7 hours. Add 5L of ice water to quench the reaction, evaporate the solvent at 30°C under vacuum, add 50L of methyl tert-butyl ether and 45L of water, extract and separate layers, wash the organic layer twice with 1M sodium dihydrogen phosphate aqueous solution, and then wash with anhydrous sulfuric acid Magnesium was dried for 2 hours, filtered, and the filtrate was concentrated and dried in vacuo to obtain 24kg colorless and transparent oily compound V 2 , the yield is about 92%.
Embodiment 2
[0045] Compound V 2 Preparation of:
[0046] Under nitrogen protection, compound V 0(10kg, 56mol) and DIPEA (50L) were added into 80L of dichloromethane, cooled to -20°C, and stirred for 10 minutes to dissolve. Slowly add trimethylchlorosilane (50 L, 168 mol) dropwise, keep the internal temperature not exceeding 10°C, return to room temperature and stir for 7 hours. Add 5L of ice water to quench the reaction, evaporate the solvent at 30°C under vacuum, add 50L of methyl tert-butyl ether and 45L of water, extract and separate layers, wash the organic layer twice with 1M sodium dihydrogen phosphate aqueous solution, and then wash with anhydrous sulfuric acid Magnesium was dried for 2 hours, filtered, and the filtrate was concentrated and dried in vacuo to obtain 18kg colorless and transparent oily compound V 2 , the yield is about 70%.
Embodiment 3
[0048] Compound V 2 Preparation of:
[0049] Under nitrogen protection, compound V 0 (10kg, 56mol) and pyridine (50L) were added into 80L of acetonitrile, cooled to -20°C, and stirred for 10 minutes to dissolve. Slowly add trimethylchlorosilane (50 L, 168 mol) dropwise, keep the internal temperature not exceeding 10°C, return to room temperature and stir for 7 hours. Add 5L of ice water to quench the reaction, evaporate the solvent at 30°C under vacuum, add 50L of methyl tert-butyl ether and 45L of water, extract and separate layers, wash the organic layer twice with 1M sodium dihydrogen phosphate aqueous solution, and then wash with anhydrous sulfuric acid Magnesium was dried for 2 hours, filtered, and the filtrate was concentrated and dried in vacuo to obtain 20kg of colorless and transparent oily compound V 2 , the yield is about 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com