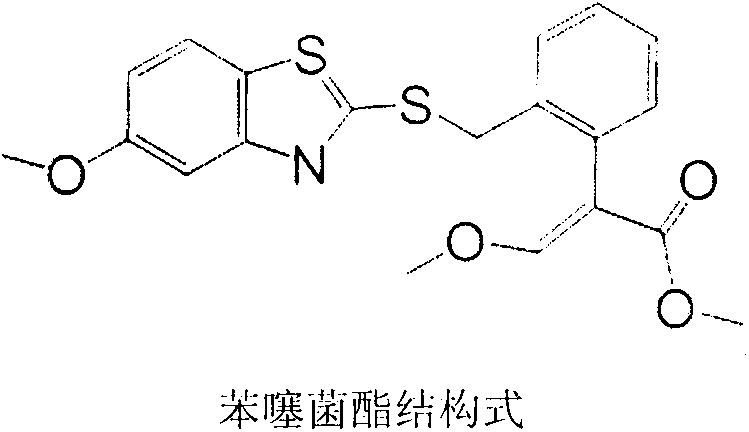
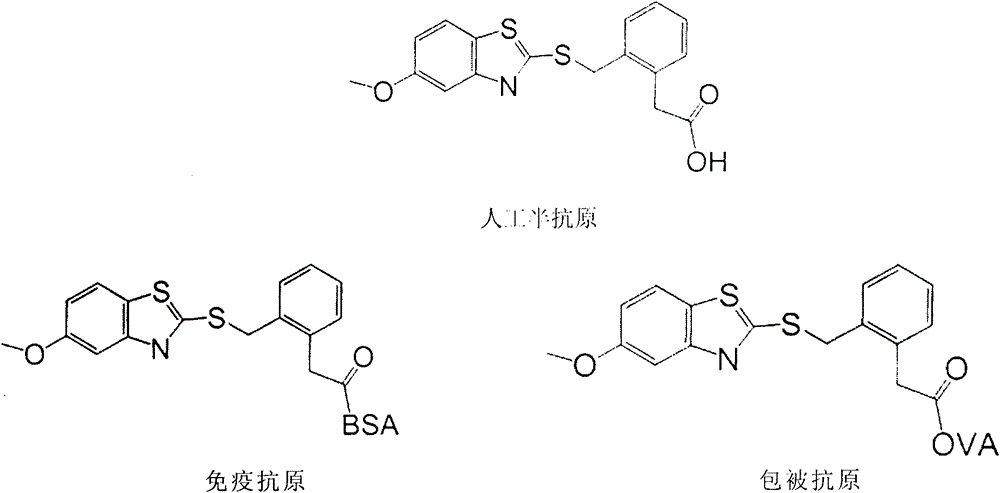
Preparation method of benzothiostrobin hapten, artificial antigen and specific antibody and application thereof
A technology of benthiazolin and antibody heavy chain, applied in chemical instruments and methods, specific peptides, animal/human proteins, etc., to achieve the effects of easy popularization, low analysis cost, and large analysis capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] 1 Synthesis of artificial hapten
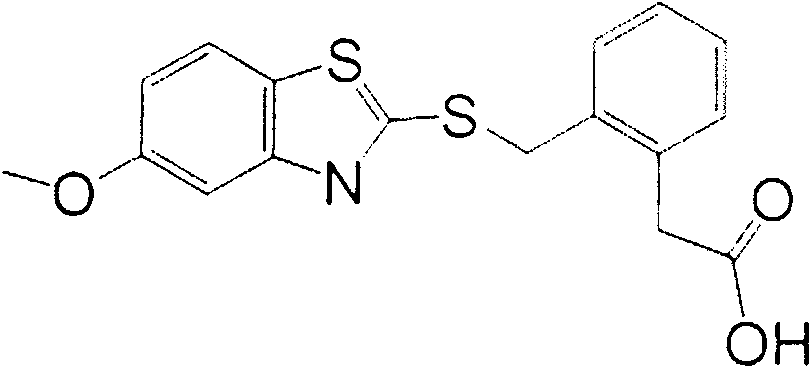
[0023] Alkaline hydrolysis of the carboxylate on the benzene ring of penthiostrobin to synthesize a hapten with a three-atom linking arm, which not only retains the characteristic group of penthiostrobin to the greatest extent, but also forms a carboxyl terminal group, which can Better for coupling to proteins. The product was purified by mass spectrometry (ESI) and H NMR spectroscopy ( 1 H-NMR) identification. The molecular structural formula of the artificial hapten of benzathystrobin is:
[0024]
[0025] 1.1 Synthesis of artificial haptens
[0026] Weigh 8.02g (0.02mol) of penthiostrobin technical substance and 30mL of tetrahydrofuran into a 100mL conical flask, then add 10.08mL of 5% lithium hydroxide solution (penthiostrobin:lithium hydroxide=1:1.05), Stir the reaction at room temperature for 4 hours, add 20 mL of water, extract three times with ethyl acetate, 40 mL each time, discard the organic phase, adjust the pH value...
PUM
| Property | Measurement | Unit |
|---|---|---|
| coefficient of variation | aaaaa | aaaaa |
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com