Preparation method of bionic injectable gelatin-silane composite medical hydrogel
An injectable and hydrogel technology, which is applied in the field of preparation of bioactive biomimetic composite medical hydrogel materials, can solve the problems of poor biocompatibility of chemical cross-linking mechanism, complex hydrogel composition, high polymer cost, etc., to achieve Good cytocompatibility, high mechanical elasticity, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
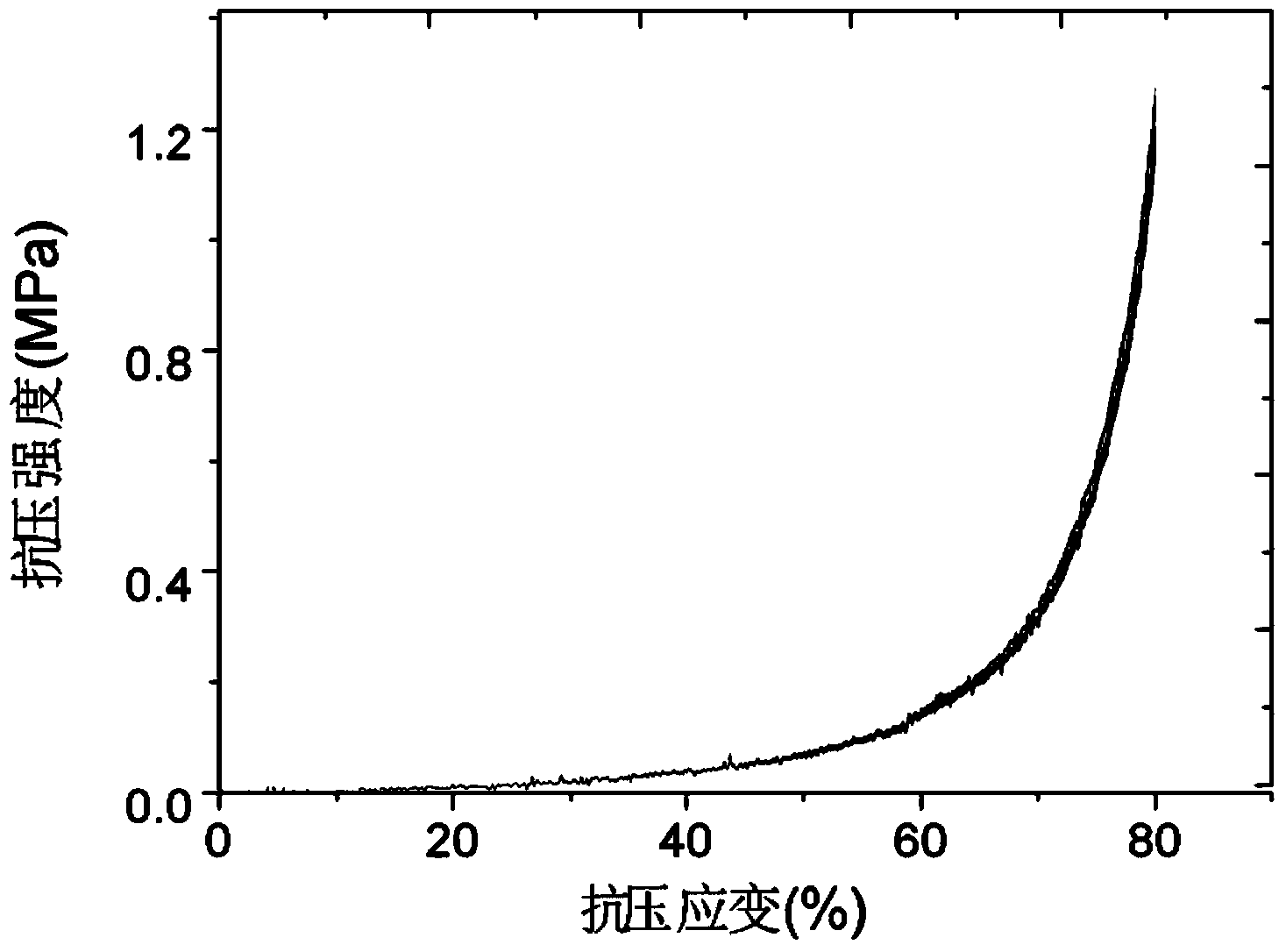
Image
Examples
Embodiment 1
[0029] (1) Preparation of gelatin solution: at 40°C, dissolve food and pharmaceutical grade gelatin powder in a phosphate buffer solution with a pH value of 7.4 and no calcium and magnesium ions (the manufacturer of the phosphate buffer solution is Shanghai Junrui Biotechnology Co., Ltd. technology company), to obtain a light yellow transparent gelatin solution; wherein, the mass concentration of gelatin in the gelatin solution is 2%, and the source of gelatin is pigskin (type A);
[0030] (2) Preparation of silane solution: Dissolve 3-glycidyl ether propyl trimethoxysilane in neutral phosphate buffer solution without calcium and magnesium ions (the manufacturer of neutral phosphate buffer solution is Shanghai Junrui Biotechnology Co., Ltd.) , to obtain a silane solution; wherein, the amount of 3-glycidyl ether propyl trimethoxysilane added to every 1 mL of neutral phosphate buffer solution was 0.05 mL;
[0031] (3) Preparation of gelatin-silane composite sol: According to 3-g...
Embodiment 2
[0034] (1) Preparation of gelatin solution: Dissolve food and pharmaceutical grade gelatin powder in a phosphate buffer solution with a pH value of 7.3 and no calcium and magnesium ions at 50°C (the manufacturer of the phosphate buffer solution is Shanghai Junrui Biotechnology Co., Ltd. technology company), to obtain a light yellow transparent gelatin solution; wherein, the mass concentration of gelatin in the gelatin solution is 20%, and the source of gelatin is pigskin (type A);
[0035] (2) Preparation of silane solution: Dissolve vinyltrimethoxysilane in neutral phosphate buffer solution without calcium and magnesium ions to obtain silane solution; among them, add vinyltrimethoxysilane to every 1mL neutral phosphate buffer solution The amount of base silane is 0.4mL;
[0036] (3) Preparation of gelatin-silane composite sol: Vinyltrimethoxysilane: gelatin is 2mL: 1g, the silane solution is added dropwise to the gelatin solution, and the hydrolysis reaction is carried out at...
Embodiment 3
[0039] (1) Preparation of gelatin solution: at 60°C, dissolve food and pharmaceutical grade gelatin powder in a phosphate buffer solution with a pH value of 7.4 and no calcium and magnesium ions (the manufacturer of the phosphate buffer solution is Shanghai Junrui Biotechnology Co., Ltd. technology company), to obtain a light yellow transparent gelatin solution; wherein, the mass concentration of gelatin in the gelatin solution is 10%, and the source of gelatin is pigskin (Type A);
[0040] (2) Preparation of silane solution: dissolve mercaptopropyl trimethoxysilane in neutral phosphate buffer solution without calcium and magnesium ions to obtain silane solution; among them, add mercaptopropyl to every 1 mL of neutral phosphate buffer solution The amount of trimethoxysilane is 0.5mL;
[0041] (3) Preparation of gelatin-silane composite sol: According to the ratio of mercaptopropyltrimethoxysilane: gelatin: 1.5mL: 1g, the silane solution was added dropwise to the gelatin soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com