Semiconductor conjugated polymer and preparation method thereof
A conjugated polymer and semiconductor technology, applied in the field of semiconductor conjugated polymers and their preparation, can solve the problems of expensive PCBM, poor compatibility, and affecting device performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of each monomer is described as follows:
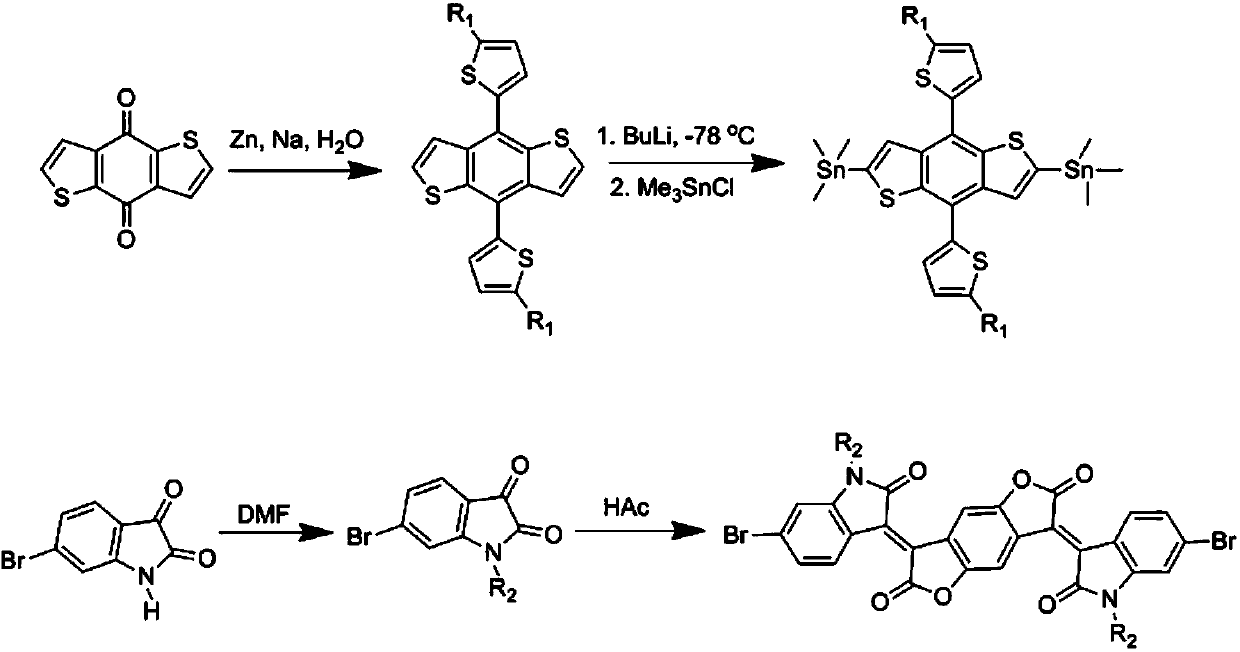
[0030] Preparation of two-dimensional conjugated benzodithiophene bistin monomer
[0031] The schematic diagram of the synthetic route of the two-dimensional conjugated benzodithiophene ditin monomer is shown in figure 2 As shown, the bistin monomer is prepared by the literature method, and the detailed preparation method can be found in the literature report (Polymer Chemistry, 2013, 4, 536-541.).
[0032] Preparation of (2-oxindol-3-ylidene)benzodifuran-dione dibromomonomers
[0033] The synthetic route of (2-oxindol-3-ylidene)benzodifuran-dione dibromomonomer is as follows figure 2 shown. For the detailed preparation method, please refer to the literature report (Chemical Communication, 2013, 49, 3790-3792.).
Embodiment 1
[0034] Embodiment 1, synthetic polymer P1
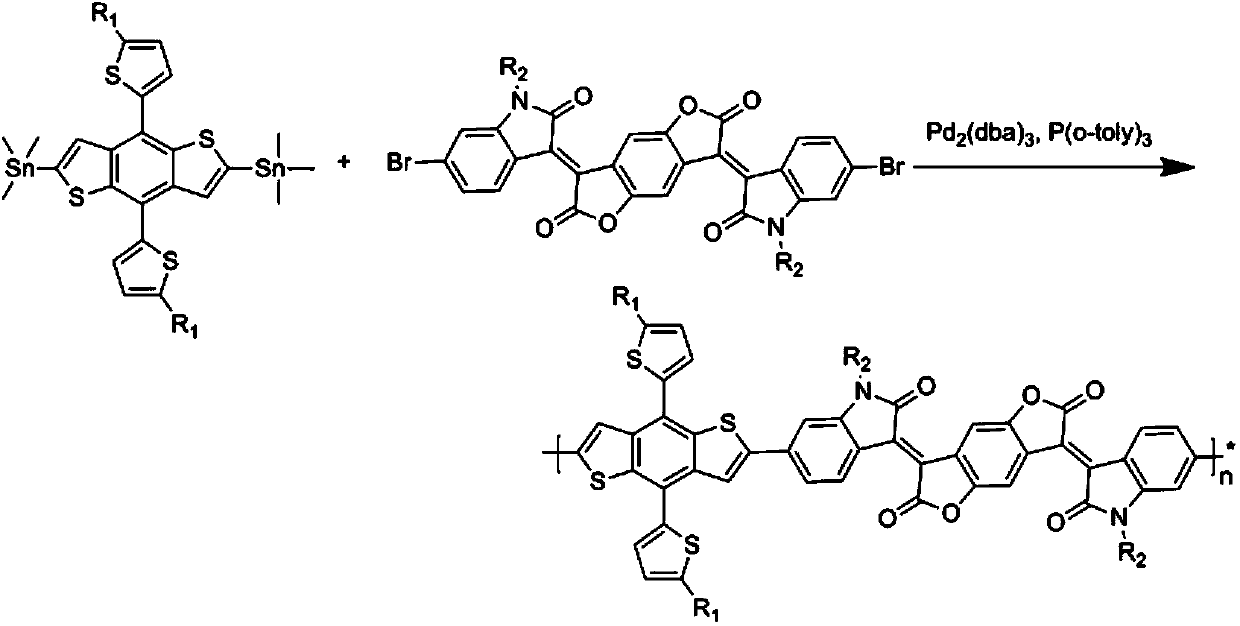
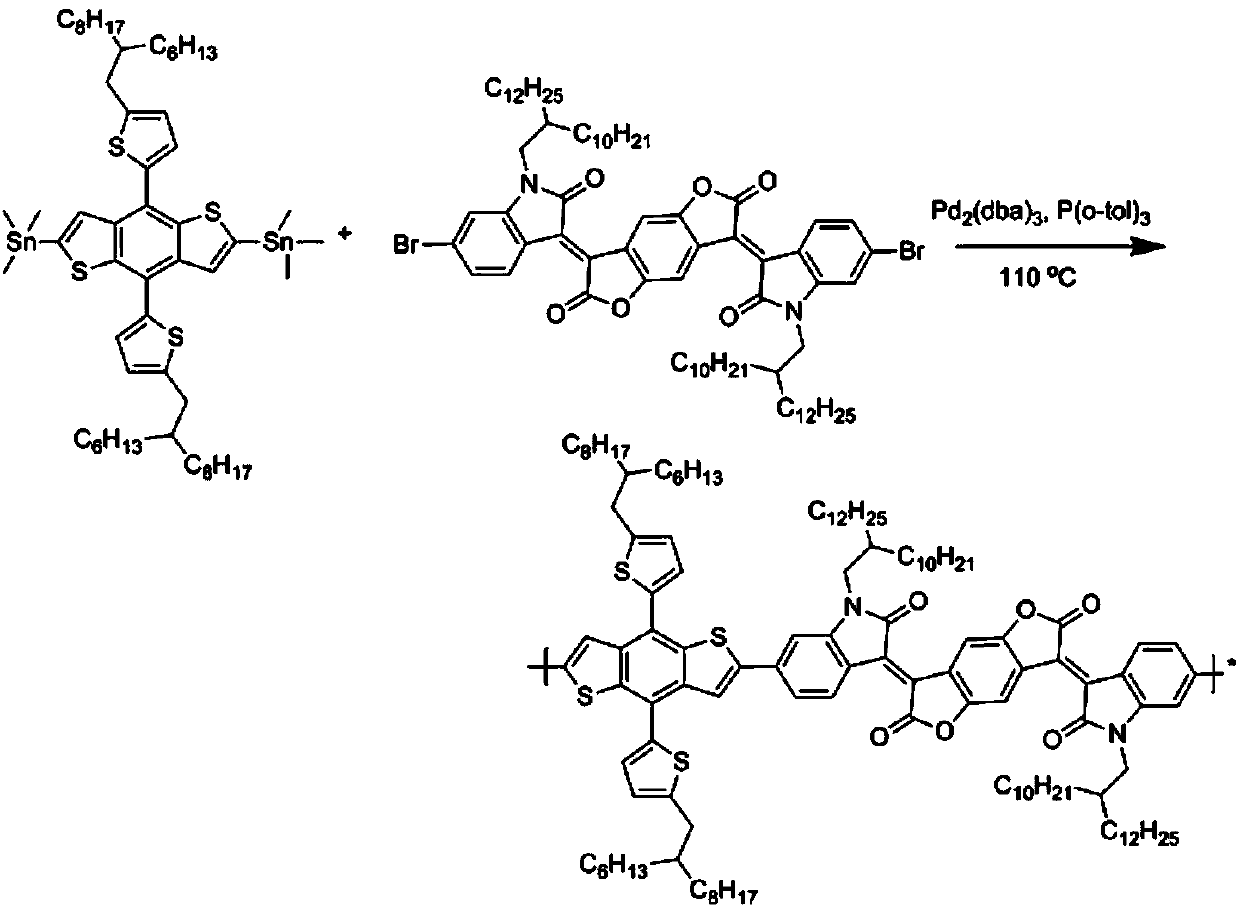
[0035] The synthetic route of polymer P1 is as follows image 3 As shown, the specific steps are: add 0.5mmol of bis-tin monomer and 0.5mmol of bis-bromine monomer (R1 and R2 are shown in Table 1) into a 100mL reaction bottle, then add 10mL of anhydrous toluene, replace with nitrogen for 40 minutes, Add 2% catalyst tris(dibenzylideneacetone) dipalladium, use tris(o-methylphenyl)phosphorus as ligand, react at 110oC for 24 hours, cool the reaction to room temperature, add 200mL of methanol to precipitate, filter the solid, respectively Soxhlet extraction with methanol and n-hexane for 24 hours, and then Soxhlet extraction with chloroform for 24 hours, and finally the liquid was rotary evaporated, methanol precipitated to obtain a black polymer.
Embodiment 2-3
[0037] The specific steps are the same as in Example 1: add 0.5 mmol of bistin monomer and 0.5 mmol of bis-bromine monomer (R1 and R2 are shown in Table 1) into a 100 mL reaction bottle, add 10 mL of anhydrous toluene, replace with nitrogen for 40 minutes, and add 2% Catalyst tris(dibenzylideneacetone)dipalladium, with tris(o-methylphenyl)phosphorus as ligand, react at 110oC for 24 hours, cool to room temperature, add 200mL of methanol to precipitate, and the solid is Soxhlet with methanol and n-hexane respectively Extracted for 24 hours, then Soxhlet extracted with chloroform for 24 hours, and finally the liquid was rotary evaporated, and methanol precipitated to obtain black polymers P2-P3, the specific structure of which is shown in Table 1.
[0038] Figure 4 The absorption spectrum of polymer P1 is given, and the absorption peak covers visible light and extends to the near-infrared region. Figure 5 The electrochemical curve of polymer P1 is given, and it can be concluded ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electron mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com