Load catalyst and preparation method and application thereof as well as method for preparing low carbon olefin by methane oxidative coupling
A supported catalyst and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. Stability, limited temperature tolerance and other issues, to achieve high activity, long-term performance and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The present invention also provides a preparation method of a supported catalyst, the method comprising: loading a metal active component on a carrier, wherein the carrier is barium titanate.
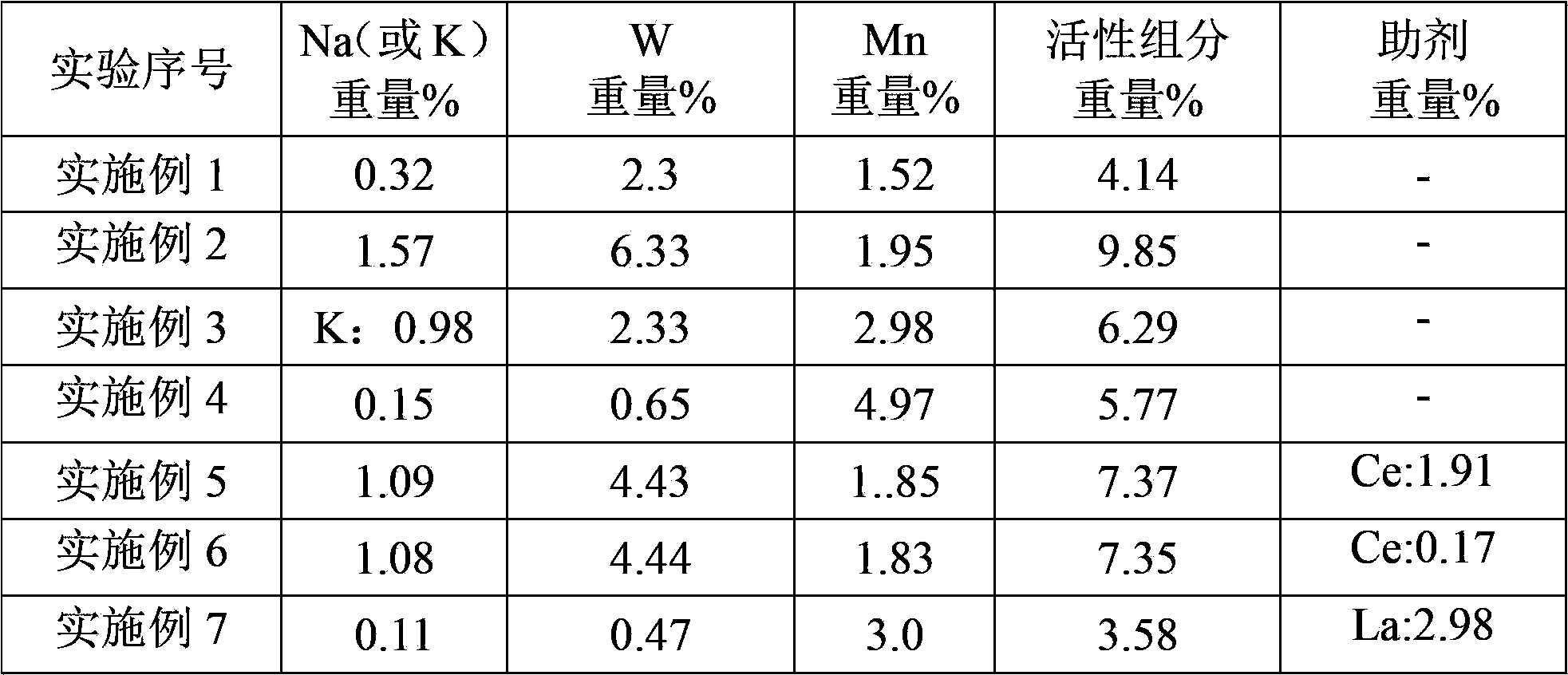
[0026] According to the preparation method of the present invention, wherein the metal active component includes a first metal active component and a second metal active component, the first metal active component is Na 2 WO 4 and / or K 2 WO 4 , the second metal active component is an oxide of Mn.
[0027] According to the present invention, the metal active component can be loaded on the support by various methods, such as ball milling or impregnation. The impregnation method can achieve the purpose of loading the metal active component on the carrier by impregnating the carrier barium titanate with an aqueous solution containing metal ions or metal acid ions containing the metal active component, and drying and roasting the impregnated solid. In the present invention, the me...
Embodiment 1
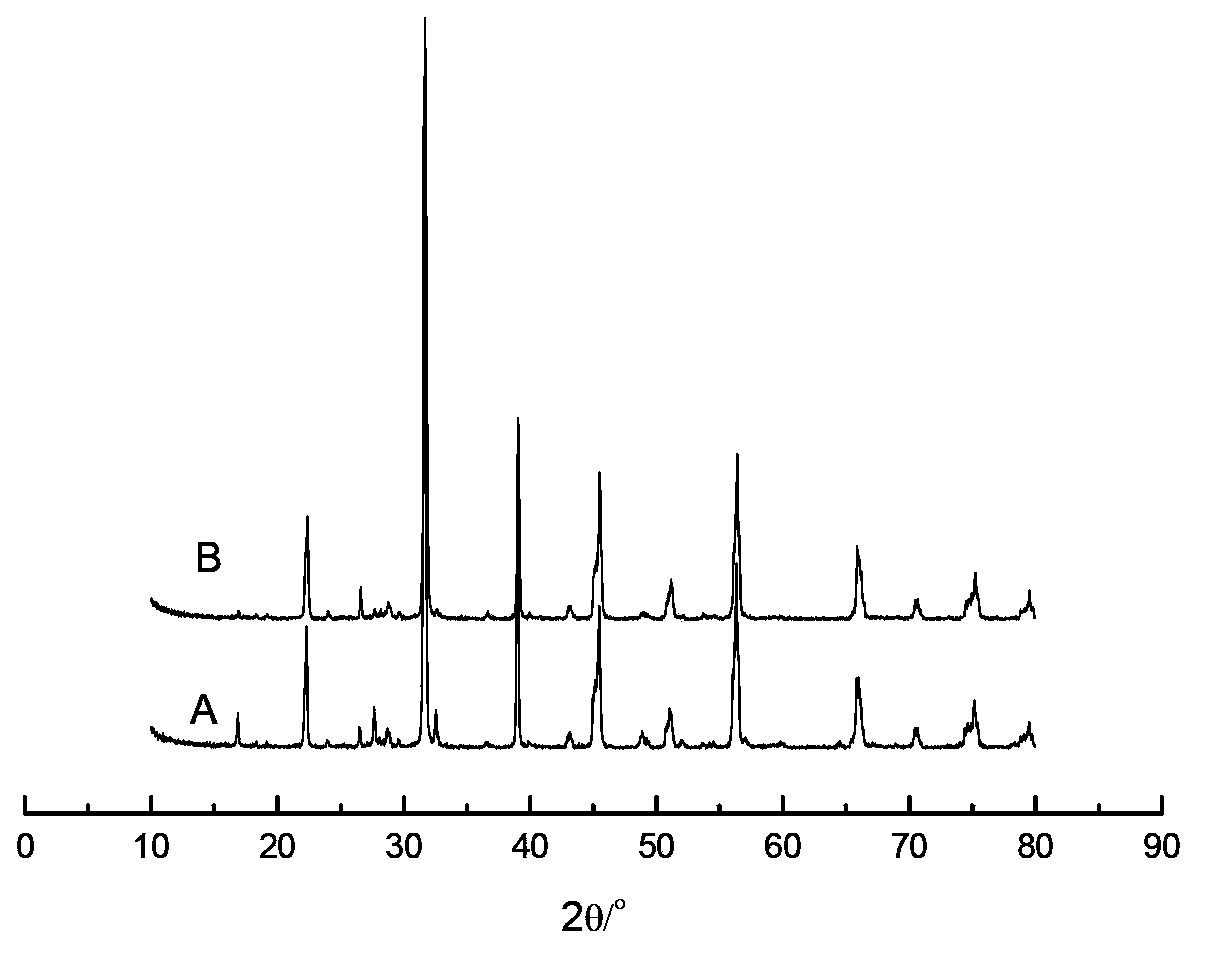
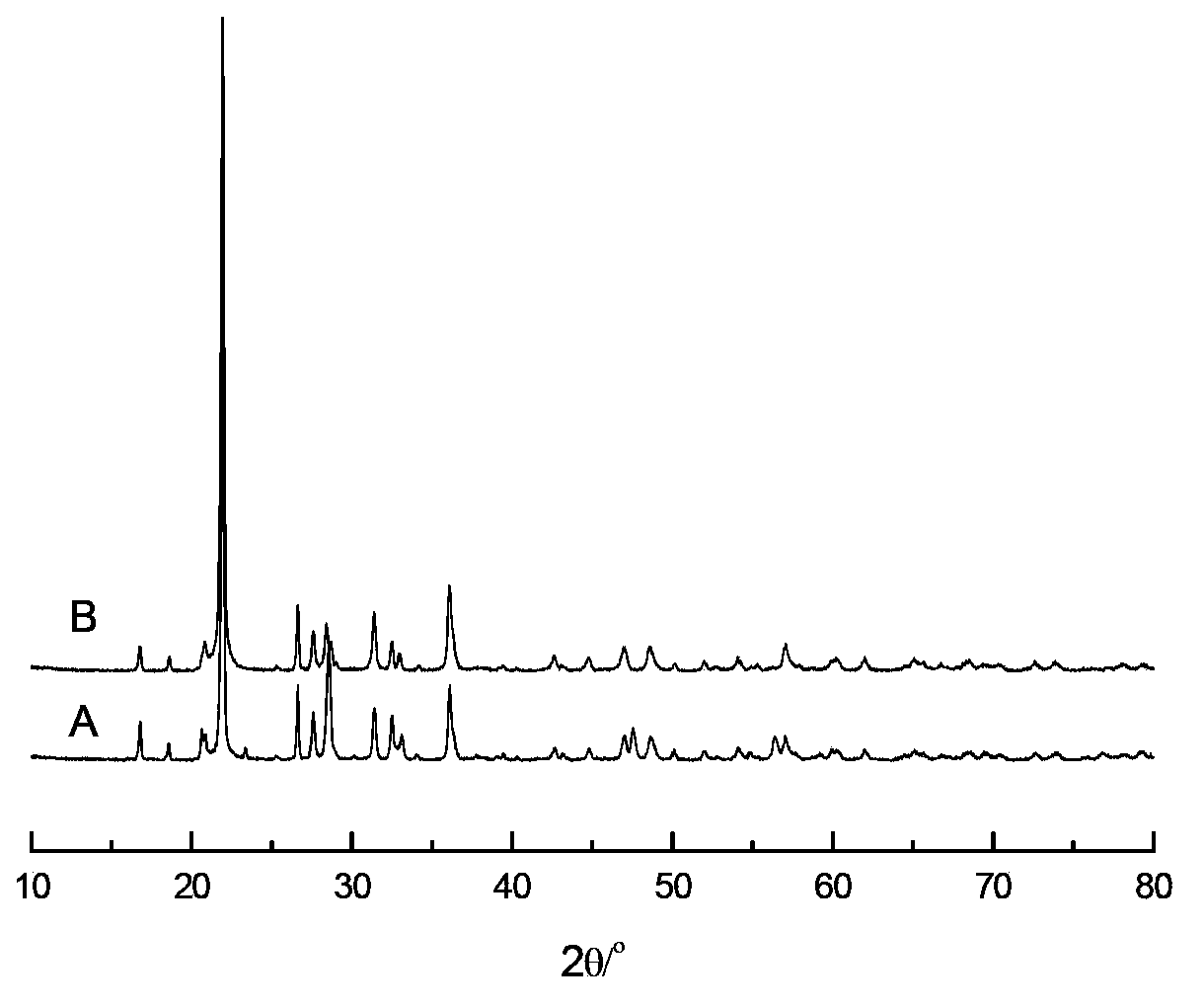
[0047] 0.18gMnCl 2 Add 25g of distilled water at 20°C, after completely dissolving, add 5g of barium titanate (the particle size of barium titanate is 2μm, and the specific surface area is 4.1m 2 / g, average pore size 30nm), stirred for 4 hours, dried at 120°C for 24 hours; then 0.04g Na 2 CO 3 Dissolve in 20°C, 25g distilled water, add the impregnated MnCl 2 Solution of barium titanate, stirred for 4 hours, dried at 120°C for 24 hours, then dissolved 0.17g of ammonium tungstate in 50°C, 25g of distilled water, added the impregnated MnCl 2 and Na 2 CO 3 in barium titanate, stirred for 2 hours, dried at 120°C for 24 hours, then calcined at 550°C for 5 hours and then heated to 850°C for 5 hours to obtain a supported catalyst. The particle size of the catalyst is 2 μm, and the specific surface area is 3.8m 2 / g, average pore diameter 25nm, the composition of metal active component in this catalyst is as shown in table 1.
[0048] The supported catalyst is used for the reac...
Embodiment 2
[0050] 0.65gMn(NO 3 ) 2 (50% by weight) aqueous solution was added to 20g of distilled water at 50°C, and 5g of barium titanate was added (the particle size of barium titanate is 5μm, and the specific surface area is 100m 2 / g, average pore diameter 155nm), stirred at constant temperature for 4 hours, dried at 120°C for 12 hours, and then 0.57gNa 2 WO 4 2H 2 O was added to 50°C, 20g distilled water, and the impregnated Mn(NO 3 ) 2 The barium titanate solution was stirred at constant temperature for 4 hours, dried at 120°C for 12 hours, then calcined at 450°C for 5 hours and then heated to 800°C for 8 hours to obtain a supported catalyst. The particle size of the catalyst was 5 μm, and the specific surface area 100m 2 / g, average pore diameter 150nm, the composition of metal active component in this catalyst is as shown in table 1.
[0051] The supported catalyst was used in the reaction of methane oxidative coupling to light olefins according to the method of Example 1,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com