Beta-hydroxyalkyl amide synthesis process
A technology of hydroxyalkylamide and synthesis process, which is applied in the field of β-hydroxyalkylamide synthesis technology, can solve the problems of low product yield, unreasonable structure of β-hydroxyalkylamide synthesis equipment, low purity, etc., and achieve reduction Energy consumption, good practical value, and the effect of speeding up the response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: a kind of β-hydroxyalkylamide synthetic technique comprises the following steps:
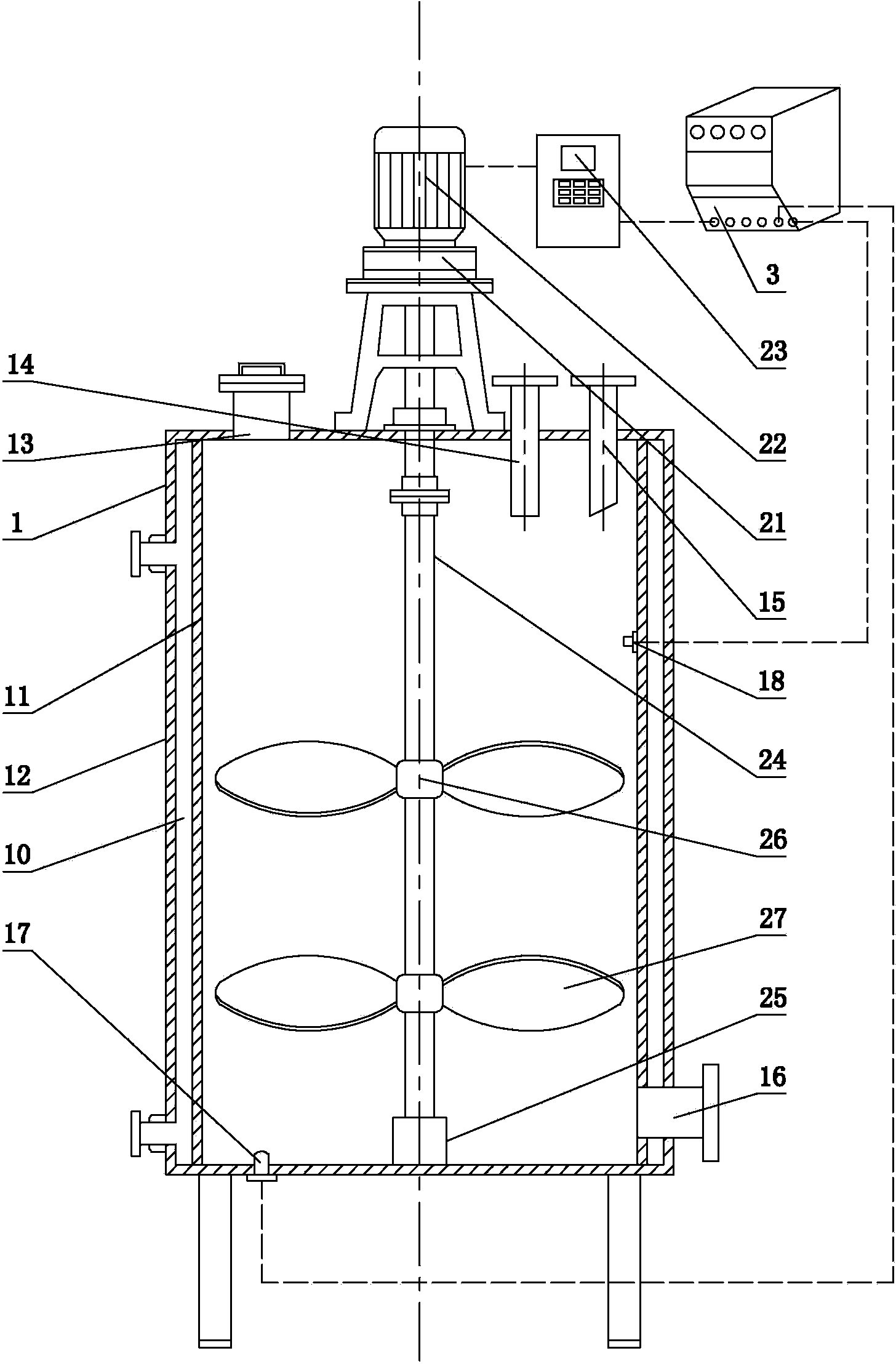
[0021] A. Put 90 parts (100 grams per part, the same below) of diethanolamine and 0.5 parts of white carbon black into the β-hydroxyalkylamide synthesis reactor, add 1.85 parts of multi-component composite catalyst to the reactor, and the multi-component composite catalyst It is made by mixing 60% sodium methoxide, 29% potassium hydroxide, 5% sodium carbonate, 5% sodium sulfite and 1% sodium citrate;
[0022] B. After the feeding is completed, first pass through the argon gas for replacement three times, then pump out the gas in the kettle, control the temperature of the material in the kettle to 80°C, and the stirring speed to 90 rpm, and then add 135.83 parts of dimethyl adipate dropwise to the reaction kettle Esters were added dropwise in 1 hour;
[0023] C. After the addition of dimethyl adipate is completed, control the temperature in the heating tank to 110°C, stir at...
Embodiment 2
[0024] Embodiment 2: a kind of β-hydroxyalkylamide synthetic technique comprises the following steps:
[0025] A. 100 parts of diethanolamine and 0.5 part of white carbon black are dropped into the β-hydroxyalkylamide synthesis reactor, and 2.05 parts of multi-component composite catalysts are added in the reactor. The multi-component composite catalyst is composed of 70% sodium methylate, 16% Potassium hydroxide, 6% sodium carbonate, 6% sodium sulfite, 2% sodium citrate are mixed;
[0026] B. After the feeding is completed, first replace it with argon for three times, then pump out the gas in the kettle, control the temperature of the material in the kettle to 85°C, and the stirring speed to 90 rpm, and then add 150.92 parts of dimethyl adipate dropwise to the reaction kettle Esters were added dropwise in 1.5 hours;
[0027] C. After the addition of dimethyl adipate is completed, control the temperature in the heating tank to 115°C, the stirring speed is 45 rpm, react for 2....
Embodiment 3
[0028] Embodiment 3: a kind of β-hydroxyalkylamide synthetic technique comprises the following steps:
[0029] A. 110 parts of diethanolamine and 0.5 part of white carbon black are dropped into the β-hydroxyalkylamide synthesis reactor, and 2.26 parts of multi-component composite catalysts are added in the reactor. The multi-component composite catalyst is composed of 74% sodium methylate, 10% Potassium hydroxide, 7% sodium carbonate, 7% sodium sulfite, and 2% sodium citrate are mixed;
[0030] B. After the feeding is completed, first replace it with argon for three times, then pump out the gas in the kettle, control the temperature of the material in the kettle to 90°C, and the stirring speed at 90 rpm, and then add 166.01 parts of dimethyl adipate dropwise to the reaction kettle Ester, added dropwise in 2 hours;
[0031] C. After the addition of dimethyl adipate is completed, control the temperature in the heating tank to 120°C, the stirring speed is 50 rpm, react for 3 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com