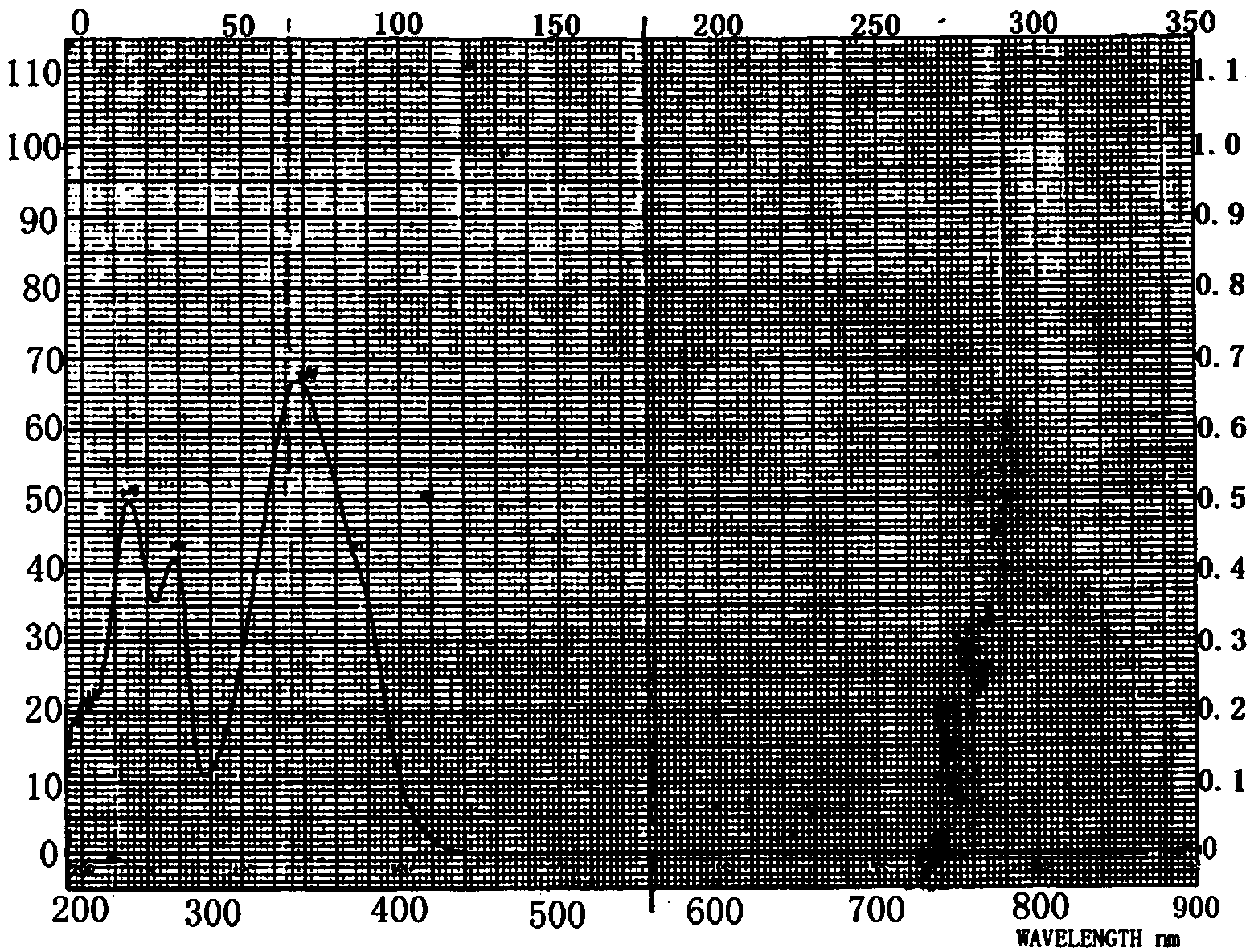
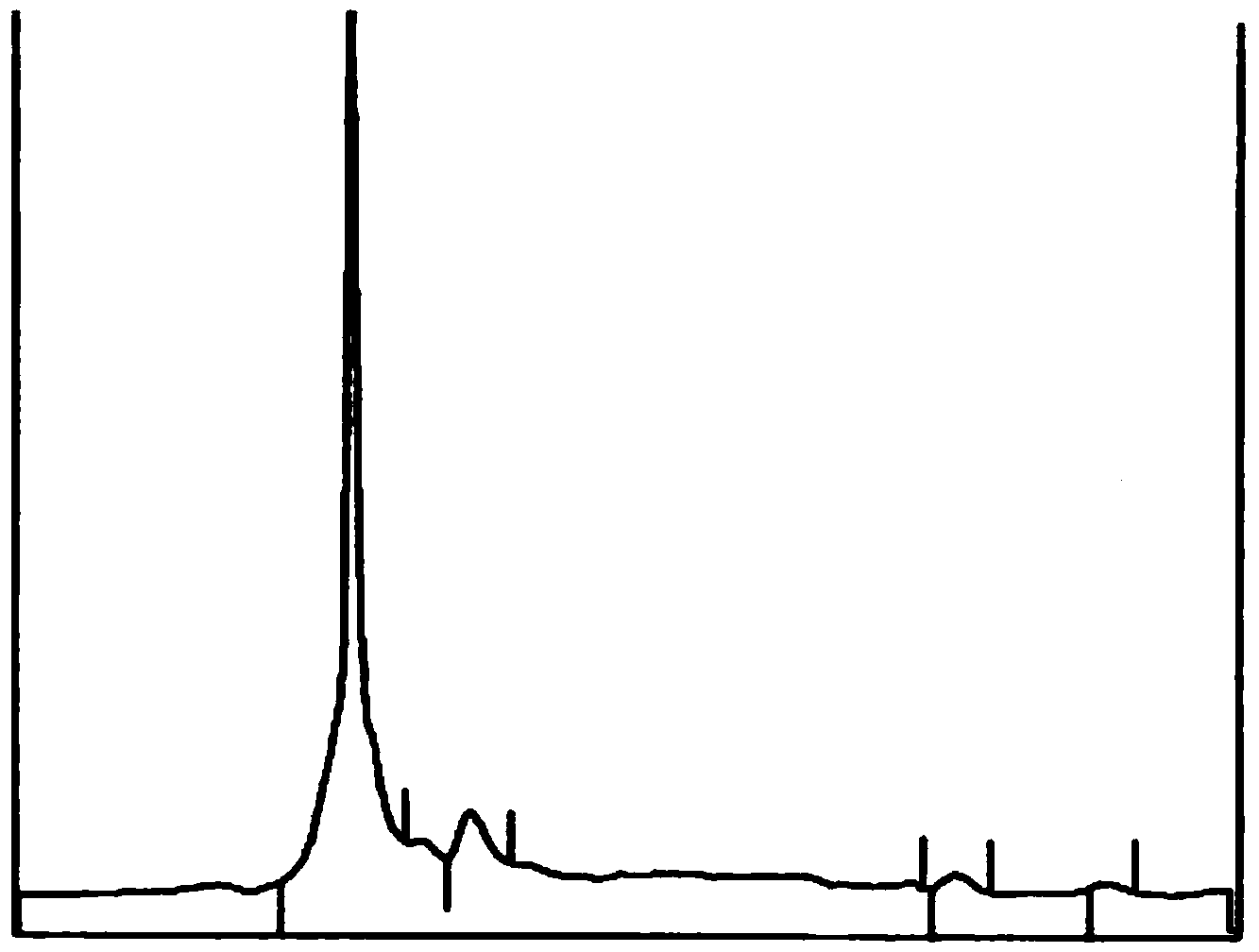
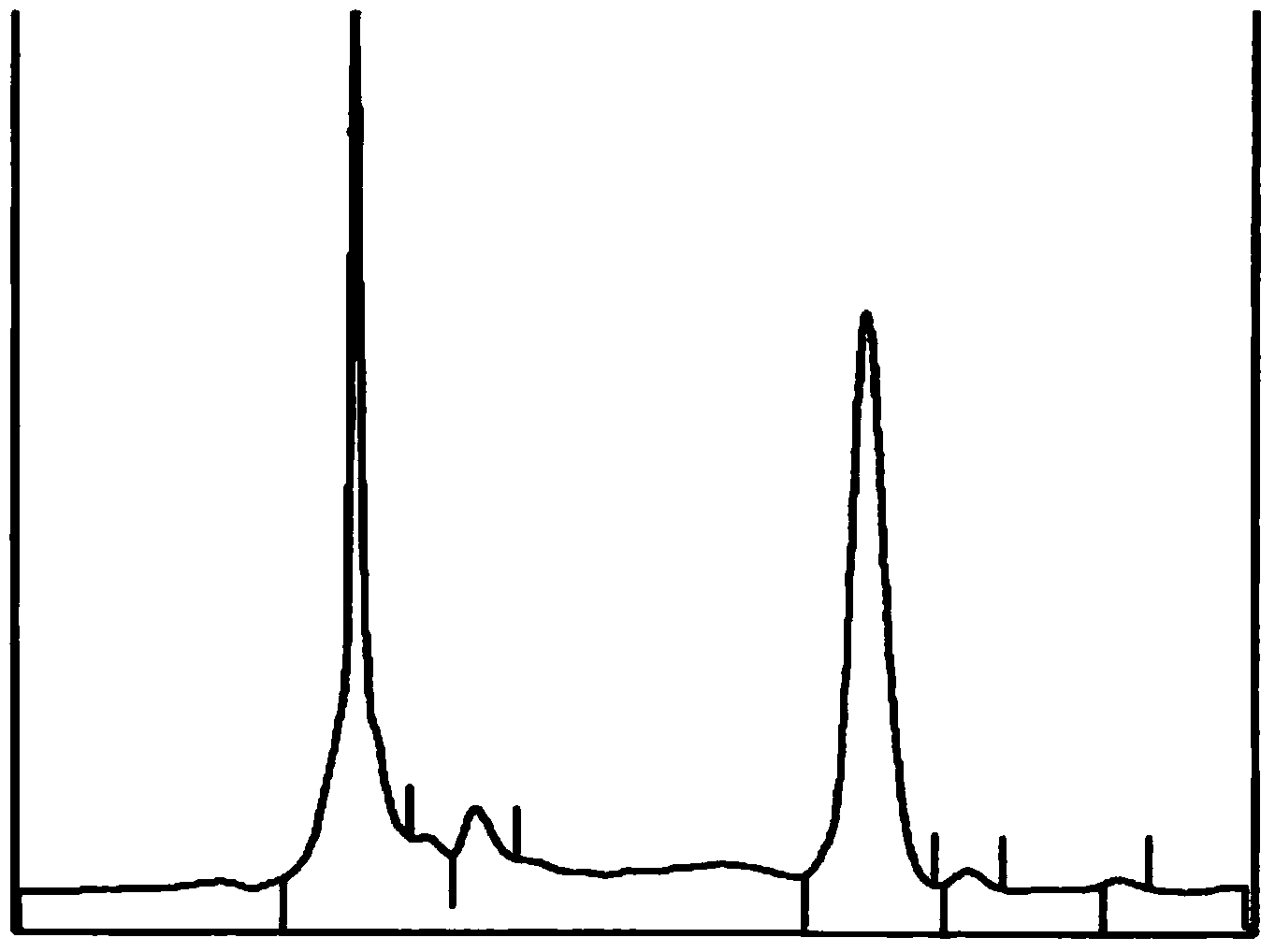
3-methyl-2-(methoxy styrene keto)-quinoxaline-1,4-dioxide, and preparation method and application thereof
A methoxy styryl ketone-based, dioxide technology, applied in the field of quinoxaline compounds, can solve the problems of poor curative effect, excessive drug residues, abuse of antibacterial drugs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] Preparation of 3-methyl-2-(o-methoxystyryl)-quinoxaline-1,4-dioxide
[0151] The structural formula is as follows:
[0152]
[0153] R is methoxy.
[0154] Its preparation method is:
[0155] 1) Synthesis of benzofurazan
[0156]
[0157] Dissolve o-nitroaniline (40g, 0.29mol) in 225ml of methanol solution, add 21g of NaOH, stir until completely dissolved, slowly add 250ml of NaClO with a concentration of 1mol / L in an ice-water bath for about half an hour, continue stirring at room temperature for 2h, TLC Follow-up detection showed that a light yellow solid was continuously precipitated, and 300ml of water was added, the solid was filtered out with suction, washed with water and dried to obtain an orange-yellow crude product, which was recrystallized from 70% ethanol to obtain yellow flaky crystals. Yield 33g, 83% yield.
[0158] 1HNMR (CDCl3) δppm: 7.81-7.90 (m, 2H), 8.55-8, 58 (d, 1H), 8.62-8.64 (d, 1H); 13CNMR (CDCl3) δppm: 113.3, 114.7, 118.2, 132.4, 133.8...
Embodiment 2
[0168] Preparation of 3-methyl-2-(m-methoxystyryl)-quinoxaline-1,4-dioxide
[0169] The structural formula is as follows:
[0170]
[0171] R is methoxy.
[0172] Its preparation method is the same as in Example 1, referring to Example 1, except that 3-methoxybenzaldehyde is used in step 3).
[0173]
[0174] Compound b is finally obtained. Light yellow powder 1.685g, yield 52%. 1H NMR (400MHz, DMSO-d6) δ: 9.70(s, 1H), 8.52(d, J=5.6Hz, 1H), 8.43(d, J=5.6Hz, 1H), 7.98(s, 2H), 7.78( d, J=16Hz, 3H), 7.24~7.10(m, 4H), 6.89(s, 1H), 2.34(s, 3H).
[0175] 13CNMR(400MHz,DMSO-d6)δ:187.28,157.76,149.19,138.93,138.57,137.66,136.71,135.31,132.40,131.43,130.05,125.30,120.15,119.64,119.64,118.82,117,10,115.40,13.99。
Embodiment 3
[0177] Preparation of 3-methyl-2-(p-methoxystyryl)-quinoxaline-1,4-dioxide
[0178] The structural formula is as follows:
[0179]
[0180] R is methoxy.
[0181] Its preparation method is basically the same as that of Example 1, except that 4-methoxybenzaldehyde is used in step 3).
[0182]
[0183] Compound c is finally obtained. Light yellow powder 1.366g, yield 42%. 1HNMR (400MHz, DMSO-d6) δ: 10.31(s, 1H), 8.52~8.43(m, 2H), 7.98(s, 2H), 7.74(d, J=14.4Hz,, 3H), 7.61(s, 2H), 7.01(d, J=14.8Hz, 1H), 6.81(s, 2H), 2.35(s, 3H).
[0184] 13CNMR (400MHz, DMSO-d6) δ: 187.35, 161.65, 150.38, 139.32, 138.00, 137.18, 135.51, 132.74, 132.00, 131.83, 129.81, 125.55, 122.58, 120.11, 117.4491, 114
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com