Carbon-phosphorus chiral dialkyl oxygen phosphine and synthesis method thereof
A synthetic method, the technology of hydroxyphenylphosphine oxide, is applied in the field of synthesis of carbon-phosphorous chiral dihydrocarbyl phosphine oxide, which can solve the cumbersome and lengthy resolution process, the low stereoselectivity of chiral phosphorus atom reaction, and the difficulty in obtaining compounds And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
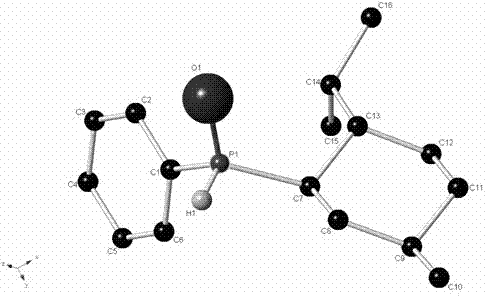
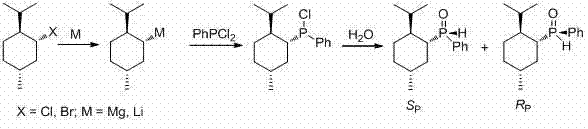
[0030] The present invention provides ( R P )-the synthetic method of menthyl phenyl phosphine oxide, its generalized steps are as follows:
[0031]
[0032] (1) Preparation of menthyl magnesium halide or menthyl lithium. According to the conventional preparation method, use ( L )-(-)-menthyl chloride or ( L )-(-)-menthyl bromide reacts with metal magnesium or metal lithium in ether solvents such as diethyl ether, tetrahydrofuran, etc. (for 0.1 molar scale reactions, use 50-300 ml of solvent) to obtain menthyl magnesium chloride or menthyl Magnesium bromide; use ( L )-(-)-menthyl chloride reacts with lithium metal in ether solvents such as diethyl ether or tetrahydrofuran, or hydrocarbon solvents such as petroleum ether, hexane, pentane or cyclohexane, etc. (0.1 molar scale reaction, Using 50-300 ml of solvent), a solution of menthyllithium was prepared.
[0033] (2) The menthyl magnesium halide reagent or lithium reagent solution prepared above is reacted with phenyl...
Embodiment 1
[0038] In a three-necked round-bottomed flask equipped with a reflux condenser and a constant pressure dropping funnel, a magnetic stirrer was placed. The whole device was fully dried in advance and filled with nitrogen, and metal magnesium chips (2.4 g, 0.1 mol) and iodine ( 5 mg). Menthyl chloride (17.4 g, 0.1 mol) was added to a constant pressure dropping funnel, and anhydrous tetrahydrofuran (200 ml dissolved) was added dropwise to the solution of menthyl chloride in tetrahydrofuran while stirring. After the reaction was initiated, the tetrahydrofuran solution of menthyl chloride was dropped in at a rate of 1 drop / second. After all of the menthyl chloride in tetrahydrofuran had been added, the reaction mixture was stirred and refluxed for 2 hours.
[0039] Freshly distilled phenylphosphine dichloride (14.1 ml, 0.1 mol) was dissolved in anhydrous tetrahydrofuran (200 ml). The solution was cooled in an ice-water bath, and the menthyl magnesium chloride solution prepared abo...
Embodiment 2
[0045] In a three-necked round-bottomed flask equipped with a reflux condenser and a constant pressure dropping funnel, a magnetic stirrer was placed. The whole device was fully dried in advance and filled with nitrogen, and metal magnesium chips (2.4 g, 0.1 mol) and iodine ( 5 mg), and immerse the round bottom flask in an oil bath. Menthyl bromide (21.8 g, 0.1 mol) was added to a constant-pressure dropping funnel, dissolved in anhydrous ether (200 ml), and the ether solution of menthyl bromide was added dropwise while stirring. After the reaction was initiated, the diethyl ether solution of menthyl bromide was dripped in at a rate of 1 drop / second. After all of the ethereal solution of menthyl bromide had been added, the reaction mixture was stirred and refluxed for 2 hours.
[0046] Dissolve freshly distilled phenylphosphine dichloride (14.1 ml, 0.1 mol) in anhydrous ether (100 ml), and cool the solution with an ice-water bath, then slowly add the menthyl magnesium bromide ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com