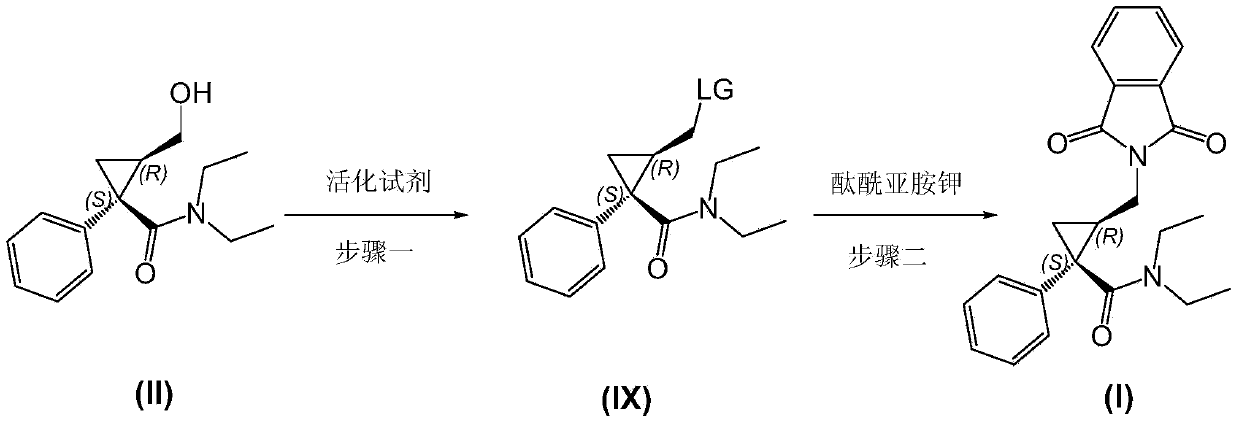
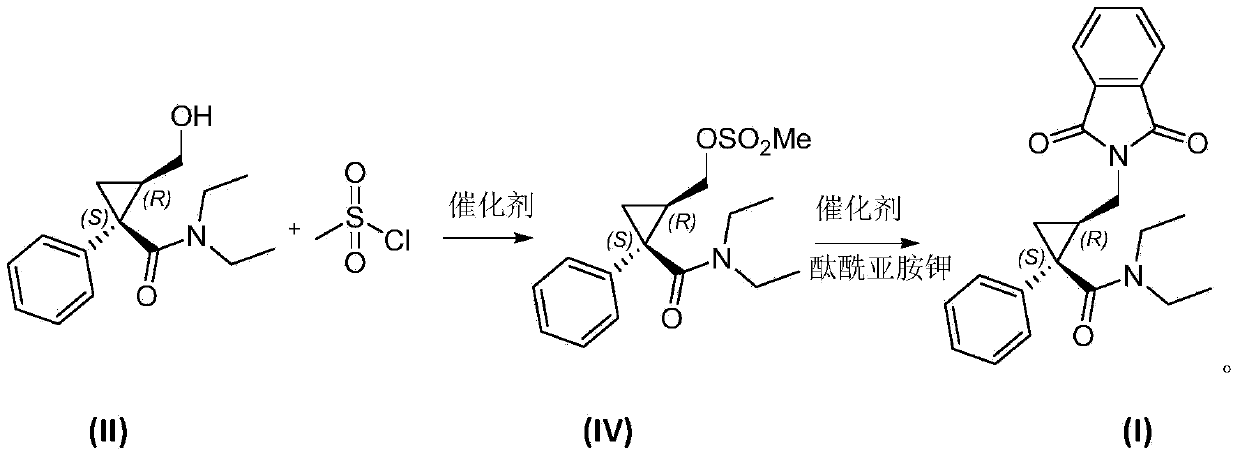
Preparation method of (1S, 2R)-1-phenyl 2-(phthalimide) methyl-N, N-diethyl-cyclopropanecarboxamide
A technology of phthalimide potassium salt and diisopropylethylamine, applied in the field of pharmaceutical synthesis, can solve the problems of many impurities, poor large-scale production efficiency, difficult solvent recovery, etc., and achieves high yield, few impurities, and easy operation easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
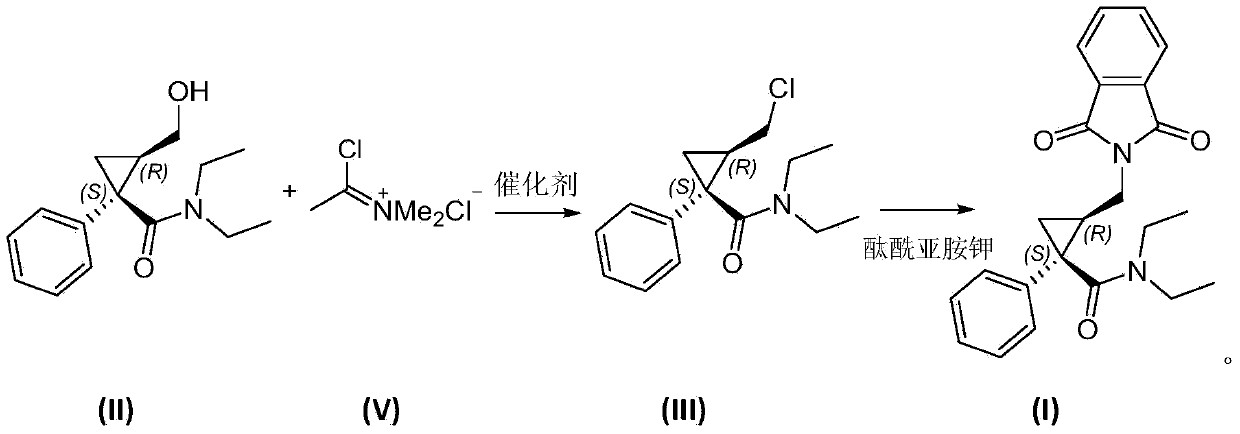
[0044] Embodiment 1 prepares chloromethylene dimethyl ammonium chloride
[0045] Add 18.8mL (242mmol) of N,N‐N,N‐dimethylformamide and 50mL of dichloromethane into a 250mL three-neck flask, stir well and cool to 0°C with brine. Slowly add 17.62mL (242mmol) of thionyl chloride dropwise to the reaction solution under the condition of nitrogen protection, so that the reaction temperature is not higher than 10°C. After the dropwise addition, the temperature is raised to 45°C and the reaction is continued for 2 hours with stirring to complete the reaction. The excess solvent was evaporated under reduced pressure (the temperature of the water bath was not higher than 50° C.), and dichloromethane (50×2 mL) was strip-evaporated twice to remove the generated acid gas. Distilled off to obtain chloromethylene dimethyl ammonium chloride.
Embodiment 2
[0046] Example 2 Preparation of (1S, 2R)-1-phenyl-2-chloromethylcyclopropane-N, N-diethylformamide (III)
[0047] Add the chloromethylene dimethyl ammonium chloride of Example 1 and 100 mL of dichloromethane into a 250 mL three-necked flask, stir until completely dissolved and cool to 0°C, then put (1S,2R)-1-phenyl- 2‐Hydroxymethylcyclopropane‐N,N‐Diethylformamide (II) 30g (121mmol) was dissolved in 50mL of dichloromethane and slowly added dropwise to the reaction solution, the reaction temperature during the dropwise addition process was not higher than 5°C, continue the reaction for 1 hour after the dropwise addition (the reaction temperature is not higher than 25°C). After the reaction, the solvent was evaporated to obtain (1S,2R)-1-phenyl-2-chloromethylcyclopropane-N,N-diethylformamide 31.5g, yield 98%; 1 H NMR(CDCl3):δ0.55(3H,t,J=7.1Hz),1.12(3H,t,J=7.4Hz),1.18(1H,dd,J=9.2Hz,J=5.2Hz),1.61 –1.66(1H,m),2.16–2.21(1H,m),3.04–3.10(1H,m),3.12–3.19(1H,m),3.50–3.70(4H,m),7.20–...
Embodiment 3
[0048] Example 3 Preparation of (1S, 2R)-1-phenyl-2-(phthalimide) methylcyclopropane-N, N-diethylformamide (I)
[0049] In a 500mL round bottom flask, add 31.5g (119mmol) of compound (III) of Example 2, 12.0g (119mmol) of triethylamine and 200mL of toluene, stir at room temperature and dissolve it completely, then add phthalimide Potassium salt 23.2g (125mmol), heat the reaction to 90°C and react for 3 hours to complete the reaction. Cool to room temperature, wash once with 5% aqueous sodium hydroxide solution (126g), set aside the aqueous phase, wash the organic phase once with 1N dilute hydrochloric acid (150mL), wash twice with saturated brine (150mL x2), and set aside the organic phase. Use 100 mL of toluene to extract the obtained four water phases once respectively, combine the toluene layers and dry, filter, and evaporate the solvent to obtain (1S,2R)-1-phenyl-2-(phthalimide)methylcyclopropane- N, N-diethylformamide 40.4g, yield 90.3%. The spectrogram data is 1 H N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com