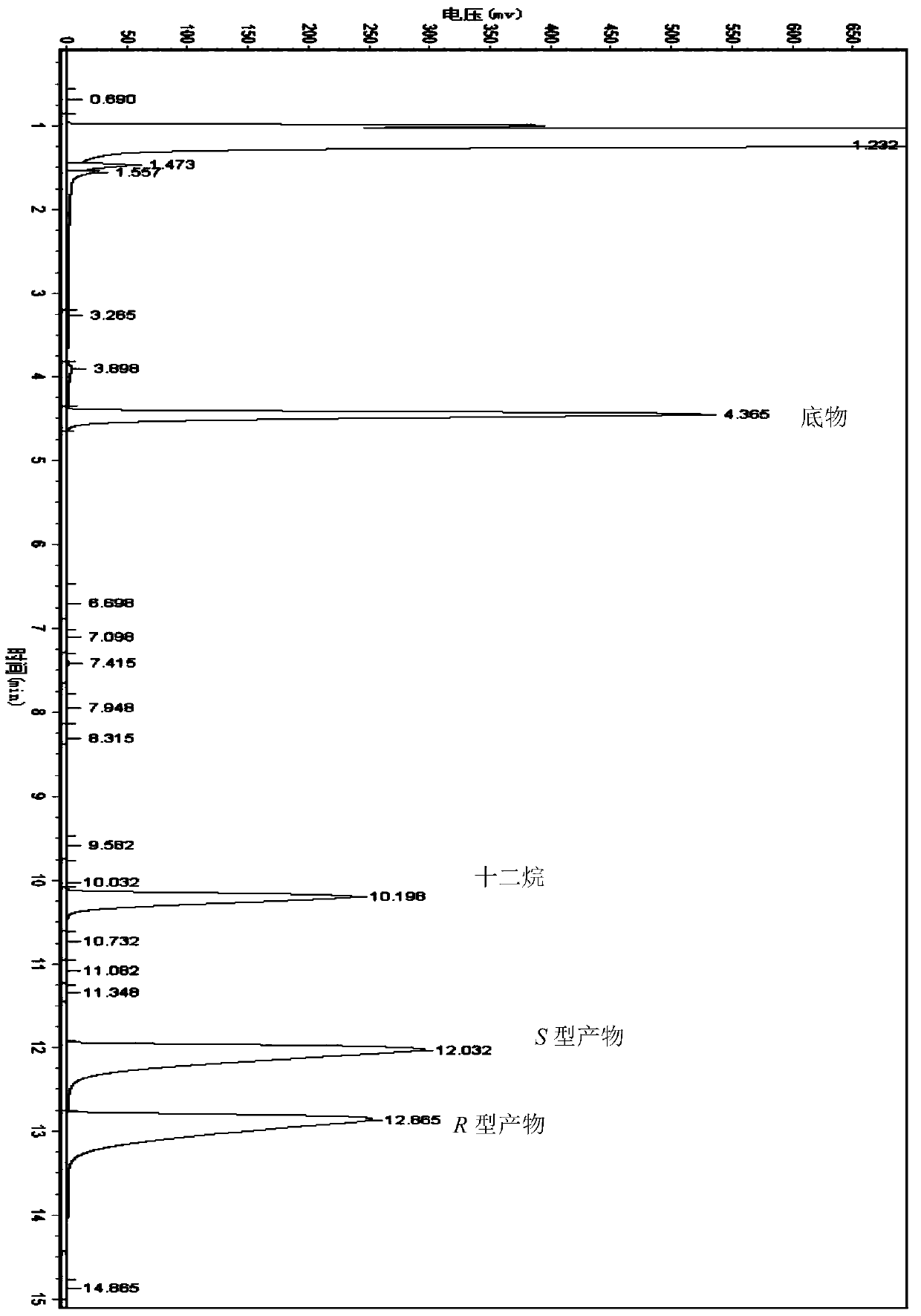
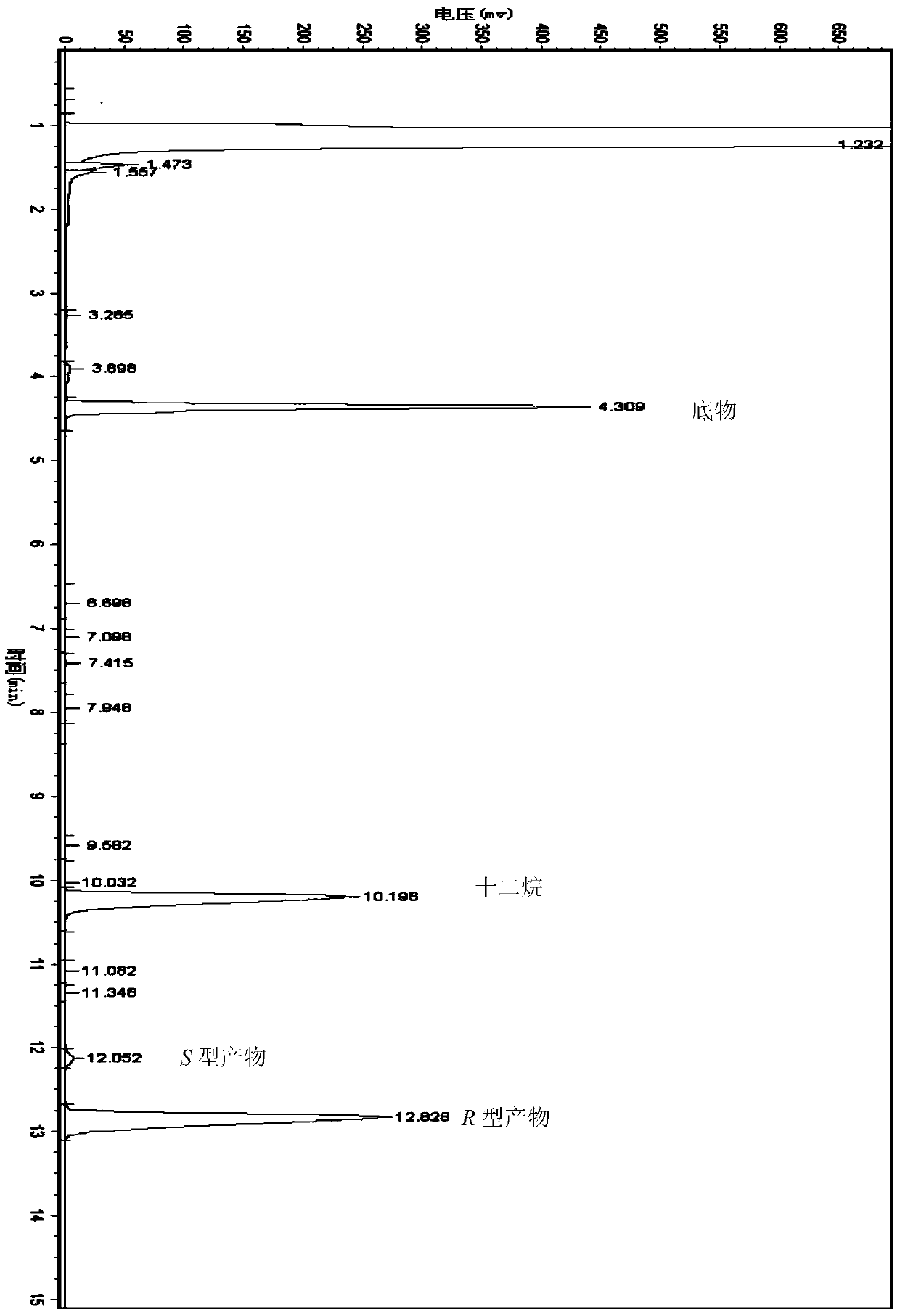
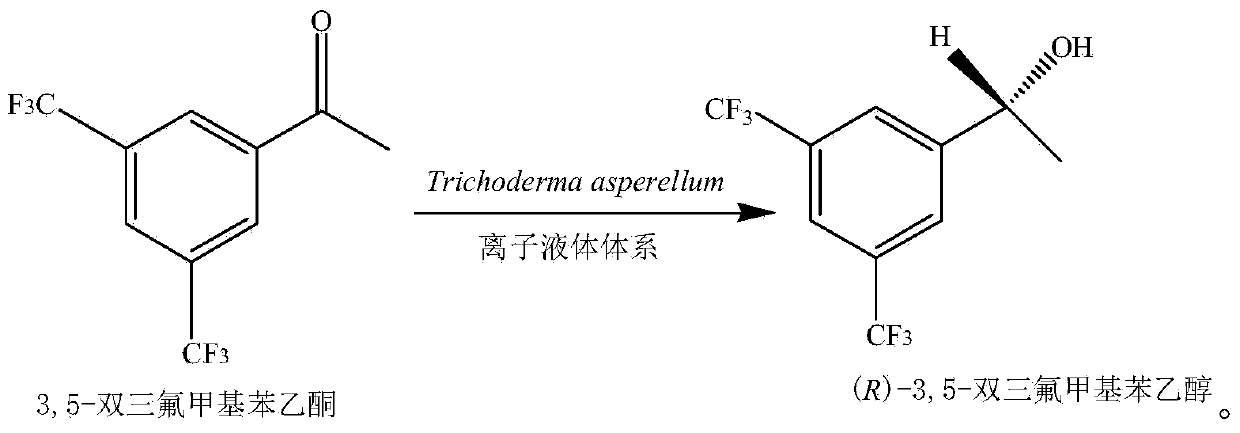
Method for preparing chiral alcohol by using reaction system containing ionic liquid
A reaction system, ionic liquid technology, applied in the field of biocatalysis preparation, can solve the problems of unfriendly environment, non-renewable, poor biodegradability and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 4
[0052] The synthesis of embodiment 1 tetramethylammonium glutamic acid ionic liquid
[0053] (1) Resin pretreatment
[0054] Put 1.2Kg201×7 type anion exchange resin in a beaker, add 1L of saturated saline to seal and soak for 24 hours, rinse it with distilled water, and then soak it in 5wt% sodium hydroxide aqueous solution with 4 times the volume of the resin for 4 hours to make it All converted into OH type, rinse the resin with distilled water until neutral.
[0055] Then soak the resin with 5wt% hydrochloric acid aqueous solution of 4 times the volume of the resin for 4 hours to make it all turn into Cl type, pour off the acid solution, and rinse the resin with distilled water until it is neutral.
[0056] Repeat the above operation 1 to 2 times (the purpose is to fully remove the impurity ions contained in the resin), and finally soak the resin with 5wt% sodium hydroxide aqueous solution of 6 times the volume of the resin for 4 hours, so that it is all converted into OH...
Embodiment 2-6
[0062] Tetramethylammonium is replaced with tetrapropylammonium, dimethyldiethylammonium, trimethylethylammonium and tetrabutylammonium respectively in embodiment 1, and glutamic acid is replaced with tyrosine, tyrosine respectively in embodiment 1. Methionine and arginine were replaced to obtain the ionic liquids described in Examples 2-6, respectively.
Embodiment 2
[0063] Embodiment 2: Tetrapropylammonium glutamic acid ionic liquid ([N 3,3,3,3 ] + [Glu] – ), Example 3: Dimethyldiethylammonium tyrosine ionic liquid ([N 1,1,2,2 ]+ [Tyr] – ), Example 4: tetramethylammonium methionine ionic liquid ([N 1,1,1,1 ] + [Met] – ), Example 5: trimethylethylammonium arginine ionic liquid ([N 1,2,1,1 ] + [Arg] – ), Example 6: tetrabutylammonium glutamate ionic liquid ([N 4,4,4,4 ] + [Glu] – ).
[0064] The tetrapropylammonium glutamic acid ionic liquid that embodiment 2 prepares: 1 H NMR (400MHz,D 2 O)δ=3.50(q,1H),2.36(q,8H),2.08(q,4H),1.65(m,2H),1.33(q,8H),0.96(t,12H);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com