Application of Dicarboxylic Acid and Its Esters
A compound and drug technology, applied in the application field of preparing neuroprotective drugs, can solve the problems of clinical application, poor effect, side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
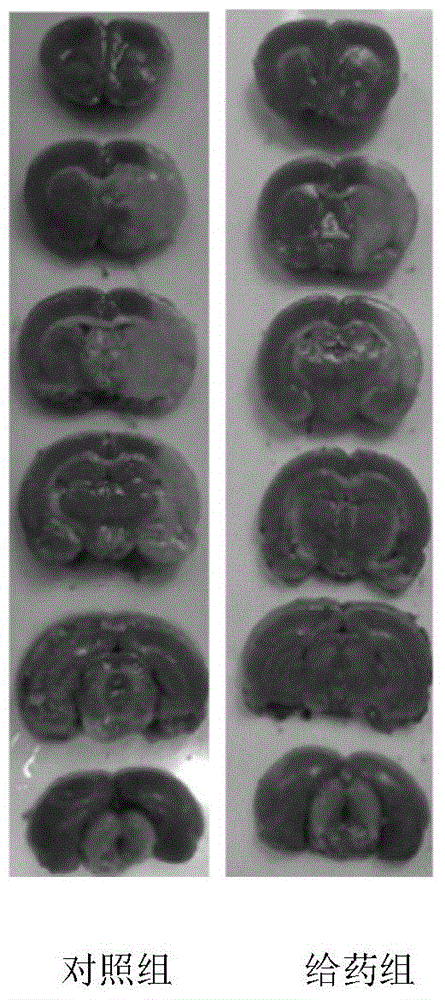
[0026] The protective effect of the compound of Example 1 on primary neuronal cell injury induced by glutamate
[0027] Experimental principle:
[0028] This experiment uses lactated dehydrogenase (Lactatedehydrogenase, LDH) assay. LDH exists in the cell, and its extracellular content is low under normal circumstances. When the cell is damaged, LDH will be released outside the cell. Therefore, the leakage rate of LDH can be used to determine the degree of cell damage. LDH can catalyze lactic acid to generate pyruvate, and the reaction of pyruvate with 2,4-dinitrophenylhydrazine to generate pyruvate dinitrophenylhydrazone, which is brown-red in alkaline solution. Enzyme activity can be obtained by colorimetry.
[0029] Extraction of primary neurons from mouse cortex:
[0030] Take ICR pregnant mice with a gestational age of 15-16 days. After cervical dislocation, the uterus is separated from the placenta, and the fetuses taken out are placed in 0.1% neocerin solution and 75% alcohol f...
Embodiment 2
[0056] Example 2 N-(2-Methoxycarbonylacetyl)-D-valine methyl ester (ZLc-002) (1) Neuroprotective effect on animal models
[0057] Preparation of N-(2-methoxycarbonylacetyl)-D-valine methyl ester (ZLc-002):
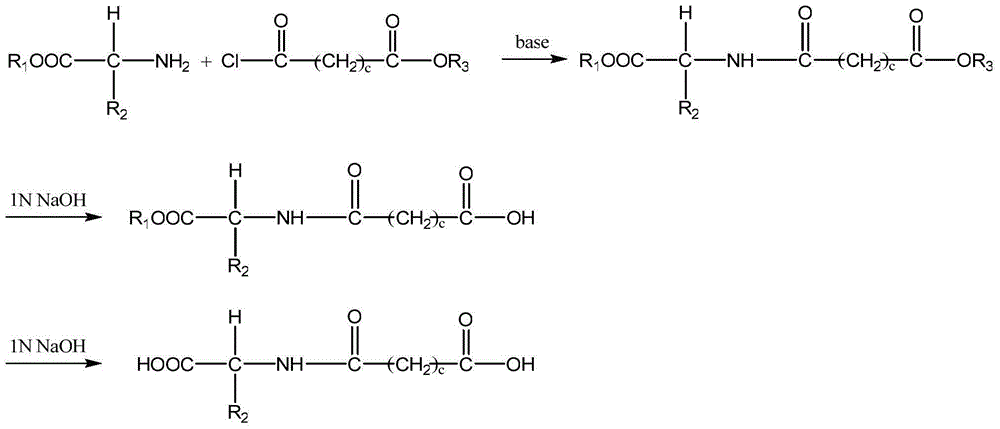
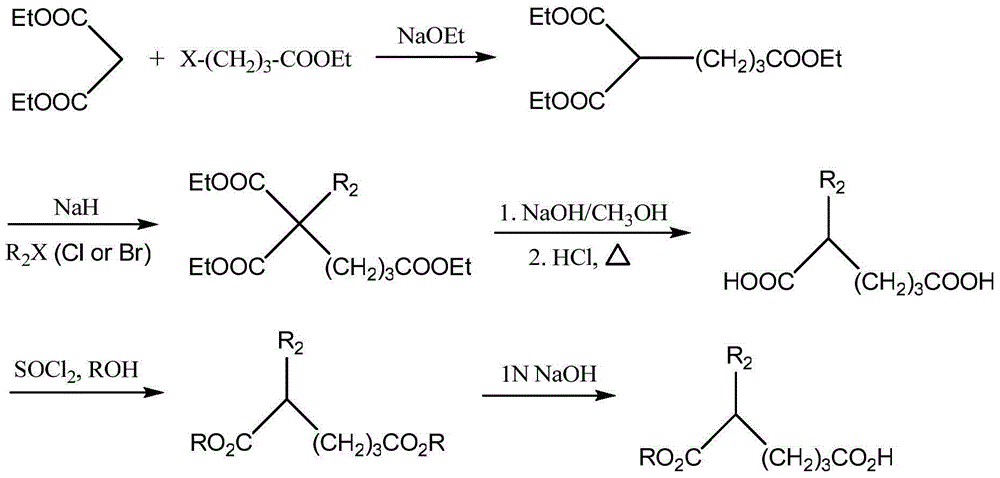
[0058] Add 1.50g (9mmol) of D-valine methyl ester hydrochloride to 35mL of dichloromethane at -15°C to dissolve, add 2.03mL (18.45mmol) of N-methylmorpholine dropwise, and stir for 5 minutes after dropping , Then add 1.02 mL (9.45 mmol) of malonate monomethyl chloride dropwise, stir for 30 minutes, and then continue stirring at 30°C for 22 hours. Stop the reaction, evaporate the solvent to dryness, add 8 mL of water, 50 mL of ethyl acetate × 4 extractions, 10% wt citric acid 25 mL × 2 washes, saturated sodium chloride wash, then 5% wt sodium bicarbonate 25 mL × 2 washes, Then washed with saturated sodium chloride, dried with anhydrous sodium sulfate, filtered, the filtrate was evaporated to dryness, and the concentrated solution was yellow. Column chromatography was performed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com