Triptolide derivatives substituted with double bonds and their preparation methods and applications
A technology of triptolide and derivatives, which is applied in the field of structural modification and activity research of active ingredients of natural medicines, and can solve the problems of increased difficulty in synthesis and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
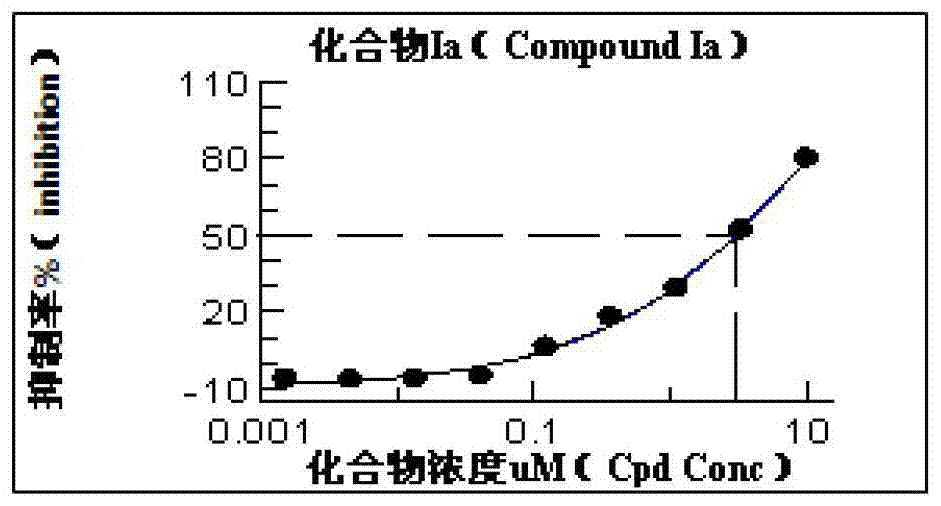
[0037] Preparation of Structural Formula (Ia) 14-Dehydroxyl-14-Methylene-5-Hydroxy Triptolide
[0038] step 1
[0039] Preparation of structural formula (2) (14S)-14β-methyl epitriptolide
[0040]
[0041] Under the protection of argon, add 10mL of anhydrous ether into the reaction flask containing magnesium chips (264mg, 11mmol), and add MeI (1.42g, 10mmol) dissolved in 5mL of anhydrous ether dropwise to the above reaction system through a constant pressure dropping funnel middle. After the addition, the dark gray reaction system was stirred at room temperature for half an hour. Take 1.37 mL of freshly prepared MeMgI format reagent solution and add dropwise to triptolide ketone 1 (90 mg, 0.25 mmmol) that has been dissolved in 8 mL of dry THF. After reacting at room temperature for 2 hours, the reaction was stopped, the reaction system was quenched with saturated ammonium chloride solution, extracted with ethyl acetate, the organic phase was washed with saturated brine, ...
Embodiment 2
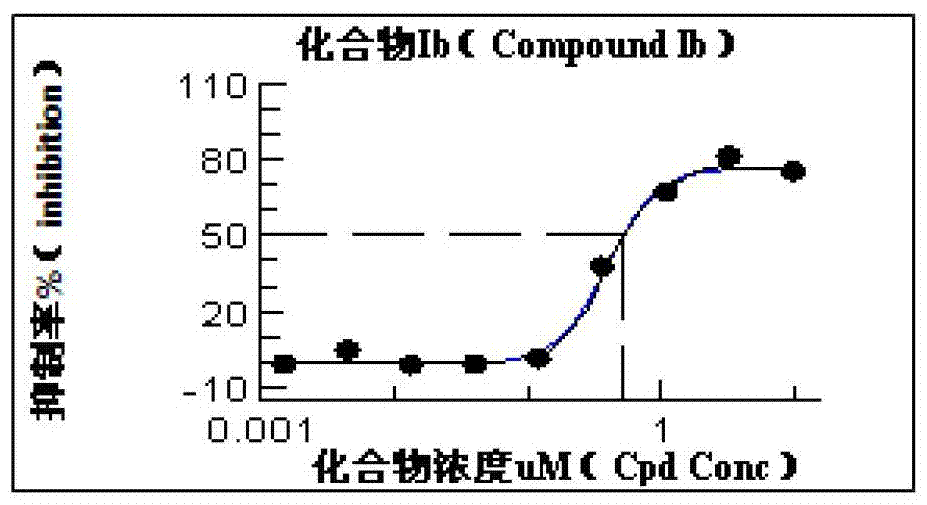
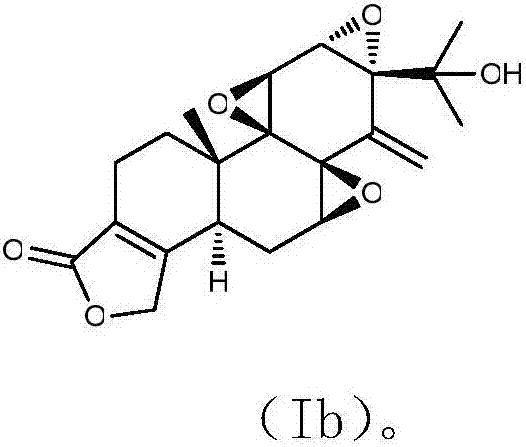
[0051] Preparation of 14-dehydroxyl-14-methylene-15-hydroxytriptolide of structural formula (Ib)
[0052] step 1
[0053] Preparation of 14-dehydroxyl-14-methylene-15-hydroxytriptolide of structural formula (Ib)
[0054]
[0055] The stock solution (50 mM) of 14-dehydroxy-14-methylene triptolide 3 was prepared in methanol, and the methanol content in the final incubation system was 0.1%. The volume of a single incubation system is 10 mL, and the medium is 100 mM phosphate buffer (PBS, pH 7.4), including rat liver microsomal protein at a final concentration of 0.5 mg / mL, 50 μM 14-dehydroxyl-14-methylene raptor Cane lactone 3 and 1mM NADPH were incubated in a water bath at 37°C for 2h. Extract 14-dehydroxyl-14-methylene triptolide 3 rat liver microsome hatching solution with ethyl acetate, concentrate under reduced pressure at 40°C to remove solvent, dissolve in 5ml methanol:water 65:35, and pass through Semi-preparative HPLC separation, collected fractions, and concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com