Metronidazole-clotrimazole-chlorhexidine acetate vaginal expansive suppository controlled-release preparation and preparation method thereof
A technology for vaginal expansion suppositories and controlled-release preparations, which is applied in the directions of suppository delivery, topical antibacterial agents, and medical preparations of inactive ingredients, etc., can solve the problem of failure to achieve zero-order release, low bioavailability, and poor solubility of active ingredients, etc. problems, to achieve the effect of preventing the outflow of liquid medicine, preventing secondary infection, and reducing irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
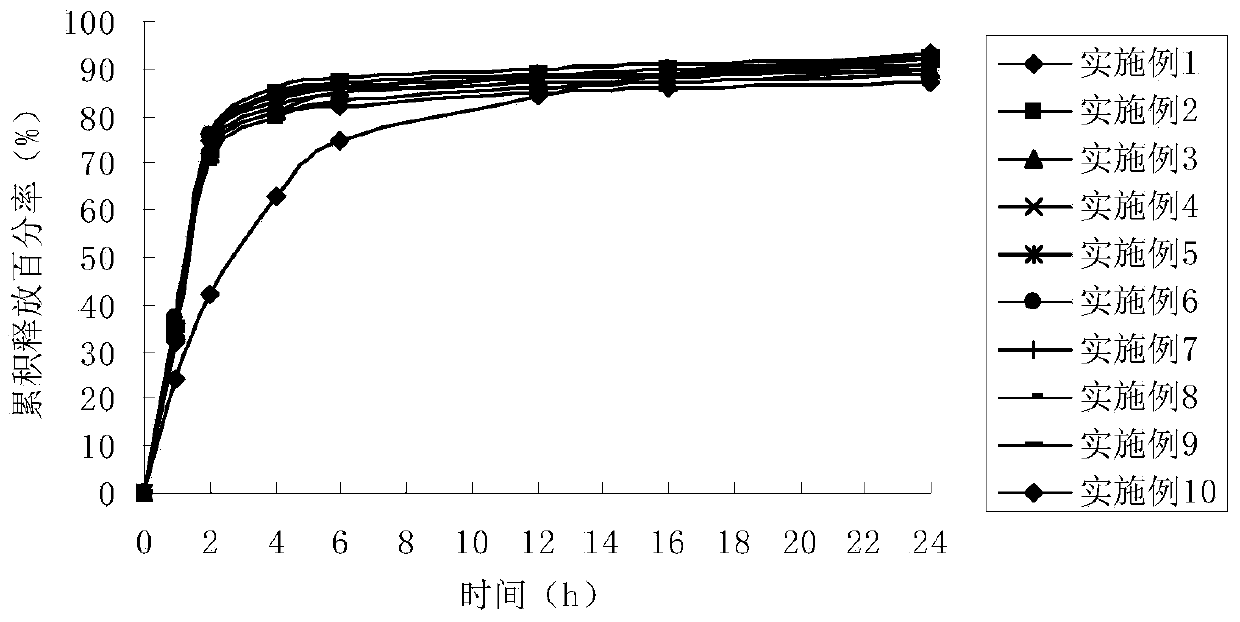
Examples
Embodiment 1
[0058]
[0059] Preparation:
[0060] 1) Preparation of the plug core coated on the surface of the expanded carrier:
[0061] a. Polyoxyethylene monostearate, Tween 60 and hydrogenated rosin oil are placed in a water bath for heating and melting to obtain a melt;
[0062] B. take metronidazole, clotrimazole and chlorhexidine acetate of recipe quantity, pulverize, add in the melt that step a makes, mix homogeneously, make mixture;
[0063] c. Pour the mixture prepared in step b into the plug mold, insert the sliver, cool and shape it, and make a plug core coated on the surface of the expanded carrier;
[0064] 2) Preparation of expansion suppository controlled-release preparation:
[0065] d. Dissolve alginic acid and polypropylene dextran in ethanol, and add diacetyl monoglyceride under stirring to prepare a homogeneous ethanol solution;
[0066] e. Place the plug core prepared in step c and coated on the surface of the expanded carrier in a coating pan, spray the coati...
Embodiment 2
[0069]
[0070] Preparation method: According to the method of Example 1, 100 spherical expansion suppository controlled-release preparations were prepared, each weighing about 1.3g, and the coating weight increased by 2%.
Embodiment 3
[0072]
[0073] Preparation:
[0074] 1) Preparation of the plug core coated on the surface of the expanded carrier:
[0075] a. Polyethylene glycol 4000 and hydrogenated castor oil are placed in a water bath for heating and melting to obtain a melt;
[0076] B. take metronidazole, clotrimazole and chlorhexidine acetate of recipe quantity, pulverize, mix with propylene glycol monopalmitic acid diester, make mixture;
[0077] c. adding the mixture prepared in step b to the melt obtained in step a, and mixing evenly to obtain a mixture;
[0078] d. Pour the mixture prepared in step c into a plug mold, insert a sliver, cool and shape, and make a plug core coated on the surface of the expanded carrier;
[0079] 2) Preparation of expansion suppository controlled-release preparation:
[0080] e. Dissolving alginic acid and polyvinyl butyral in ethanol, adding dipropyl sebacate under stirring to make ethanol homogeneous solution;
[0081] f. Place the plug core prepared in st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com