Aminopyridine type acetyl synanthrin and preparation method thereof
A technology of aminopyridine acetyl inulin and aminopyridine, which is applied in the field of daily chemicals and achieves the effects of easy promotion, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthetic route of aminopyridine chloroacetyl inulin is as follows.
[0035]
[0036] Wherein R is a pyridine compound containing an amino group, and the average value range of n is 10-35.
[0037] In this example, the target compound aminopyridine acetylinulin was synthesized according to the above synthetic route.
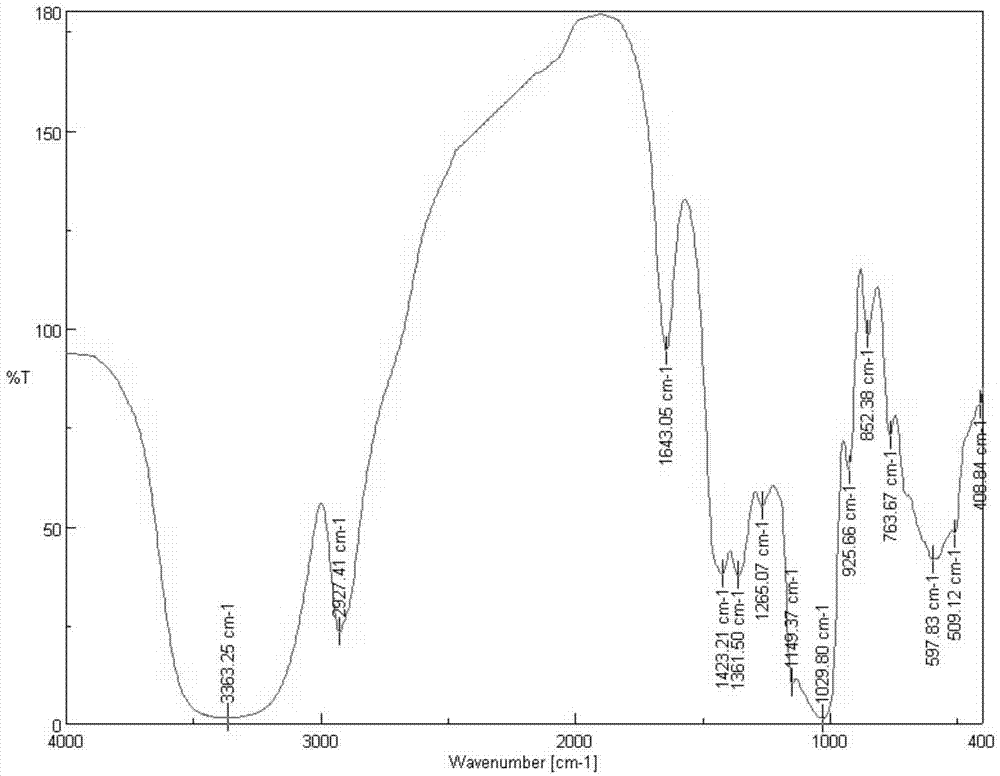
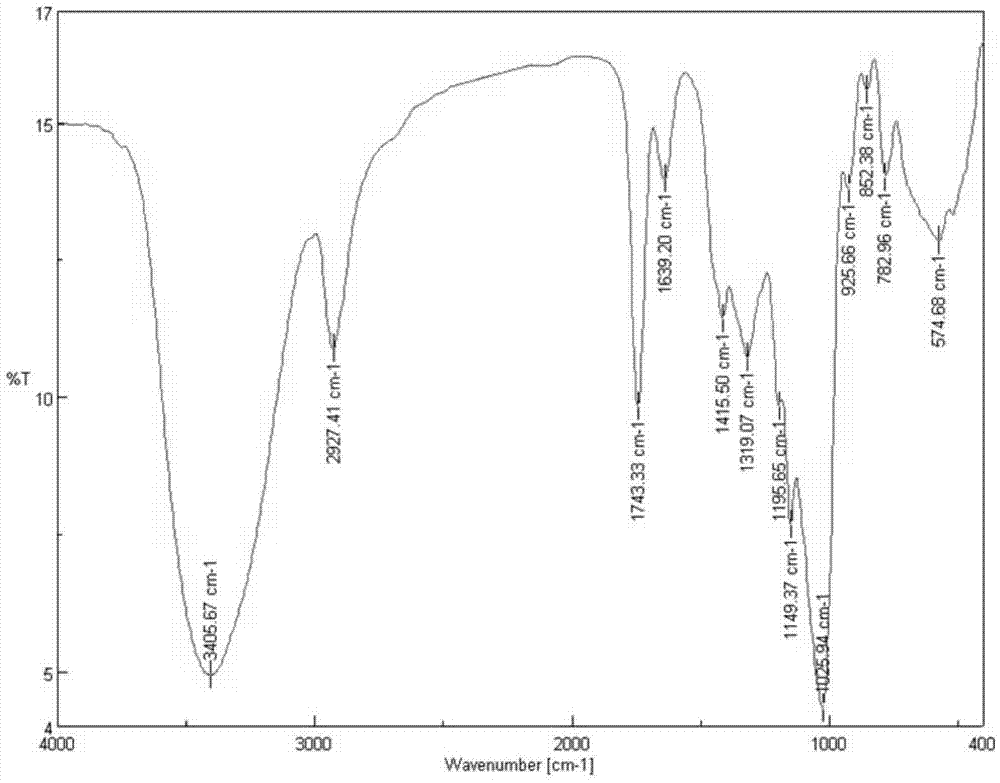
[0038] 1) Preparation of chloroacetyl inulin: 3.2g inulin (see figure 1 ) was dissolved in 100mL distilled water at room temperature for 20min, and after adding 4.4mL chloroacetyl chloride, reacted at room temperature for 24h. , to obtain the cyan product chloroacetyl inulin (see figure 2 ) 2.5g, set aside. Chloroacetyl inulin is blue powder or lumpy solid, easily soluble in cold and boiling water and various organic reagents without being pasty, yellow transparent liquid.
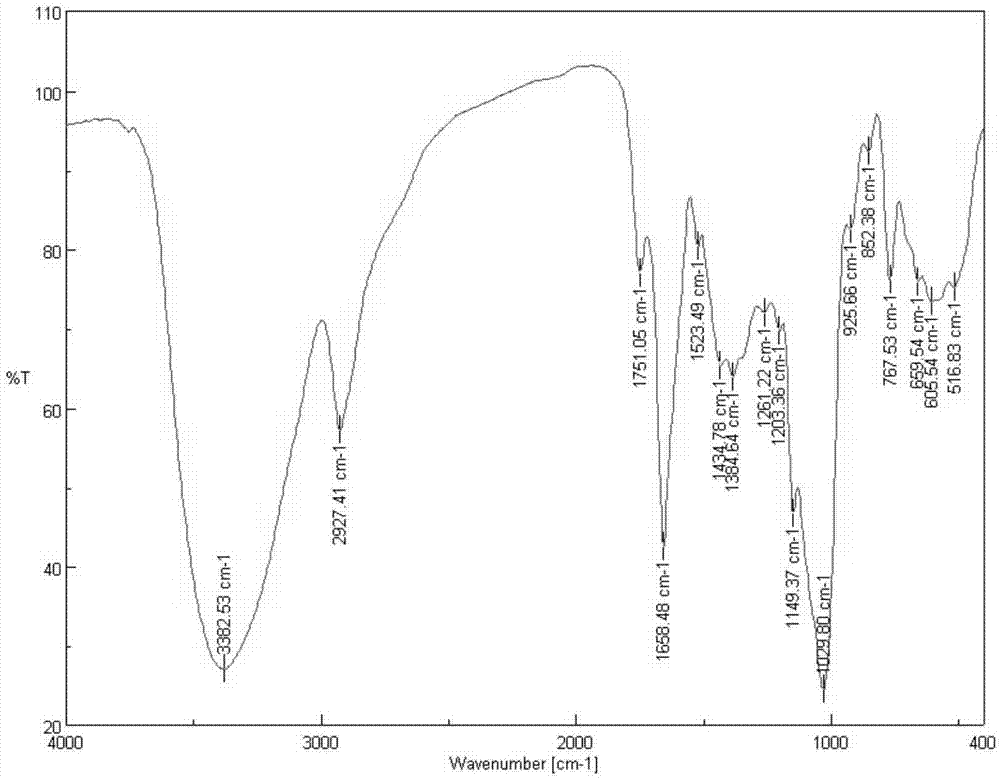
[0039] 2) Preparation of 4-aminopyridine acetyl inulin: 0.5 g of chloroacetyl inulin (see figure 2 ) into 20mL DMF (N,N-dimethylformamide), then add 2-3 times the molar a...
Embodiment 2
[0043] The difference from Example 1 is:
[0044] 1) Preparation of chloroacetyl inulin: 3.2g inulin (see figure 1 ) was added to 100mL distilled water and dissolved at room temperature for 20min, after adding 4.4mL chloroacetyl chloride, reacted at room temperature for 12h, after the reaction was completed, concentrated by rotary evaporation under reduced pressure, and precipitated with 50mL acetone after cooling, suction filtered, washed, and vacuum freeze-dried , to obtain the cyan product chloroacetyl inulin (see figure 2 ) 1.8g, set aside.
[0045] 2) Preparation of 4-aminopyridine acetyl inulin: 0.5 g of chloroacetyl inulin (see figure 2 ) into 20mL DMF (N,N-dimethylformamide), then add 1.5 times the molar amount of aminopyridine compound, and react at 50°C for 12h. After the reaction, precipitate with acetone, suction filter, wash, and dialyze 36h, vacuum freeze-drying to obtain the target product. The target product is a blocky solid which is readily soluble in w...
Embodiment 3
[0047] The difference from Example 1 is:
[0048] 1) Preparation of chloroacetyl inulin: 3.2g inulin (see figure 1 ) was dissolved in 50mL distilled water at room temperature for 20min, added 4.4mL chloroacetyl chloride, and reacted at room temperature for 8h. , to obtain the cyan product acetyl inulin (see figure 2 ) 1.2g, set aside.
[0049] 2) Preparation of 4-aminopyridine acetyl inulin: 0.5 g of chloroacetyl inulin (see figure 2 ) into 20mL DMF (N,N-dimethylformamide), then add 1 times the molar amount of aminopyridine compound, and react at 50°C for 24h. After the reaction, precipitate with acetone, suction filter, wash, and dialyze 36h, vacuum freeze-drying to obtain the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com