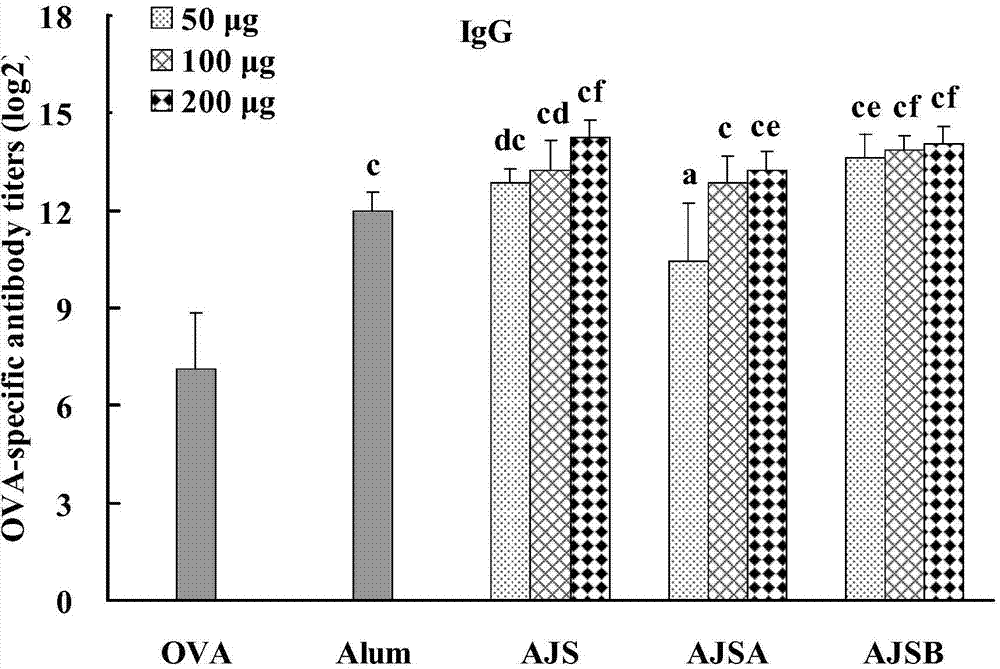
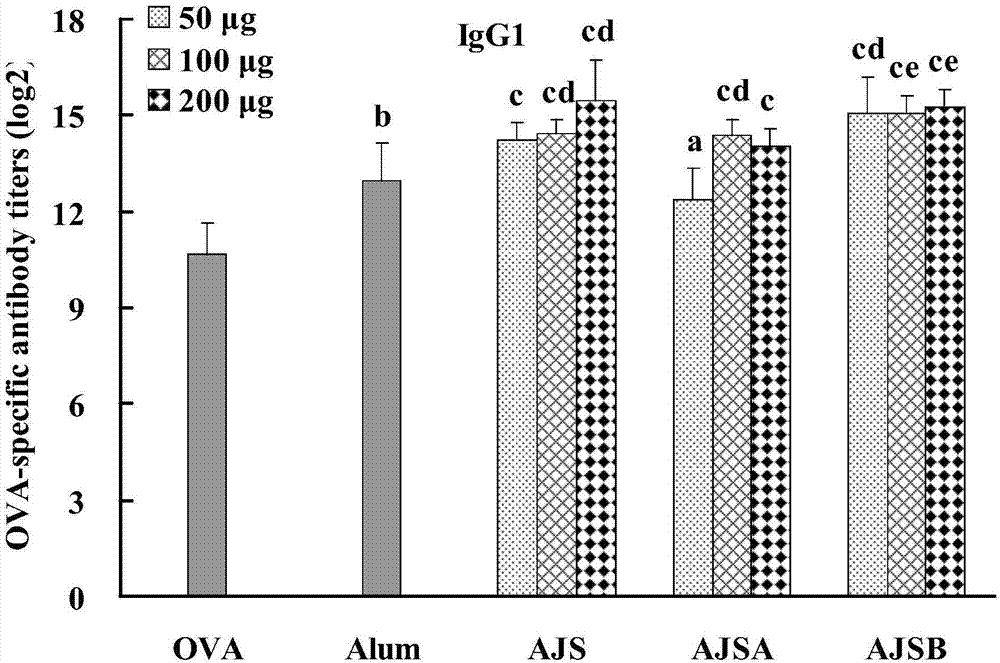
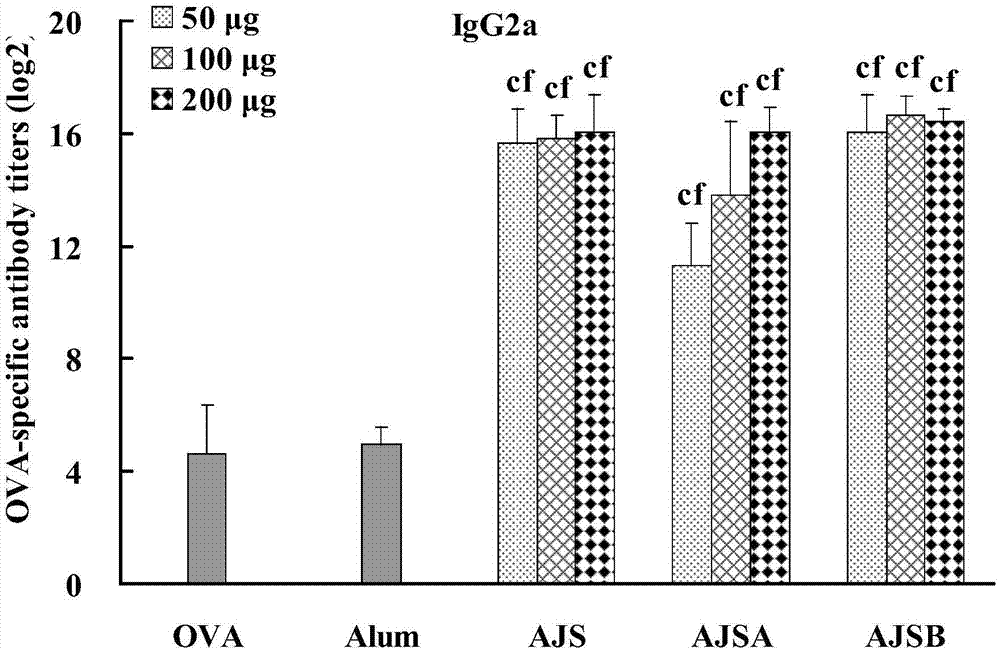
Cortex albiziae saponin adjuvant and preparation method thereof, as well as vaccine preparation containing adjuvant and application thereof
A technology for saponin of Albizia Julibrissin and Albizia Julibrissin bark, which is applied in the application field of preparing vaccine preparations, can solve problems such as adverse reactions and poisoning, complex chemical structure, formation of granulomas, etc., and achieves easy quality control, simple preparation process, and low hemolysis. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: Preparation of Albizia Julibrissin
[0045] Preparation of Total Saponins from Albizia Julibis
[0046] Medicinal platycodon grandiflorum powder 1 kg, reflux extraction with 70% ethanol for 3 times, each time for 2 hours, filter, combine filtrate, recover ethanol under reduced pressure, add appropriate amount of water to suspend the extract, extract with ether until the ether liquid is nearly colorless . The aqueous layer was extracted with water-saturated n-butanol until the extract was nearly colorless. The extracts were combined, recovered under reduced pressure, and concentrated to obtain the alcohol extract. The alcohol extract was passed through a D101 macroporous adsorption resin column, and then eluted with water and 75% ethanol in sequence, and the eluate was checked by thin-layer chromatography. The fractions with the same spots were combined, recovered under reduced pressure, concentrated, and freeze-dried to obtain acacia bark saponins. Crud...
Embodiment 2
[0053] Example 2 Hepatitis B vaccine preparation containing saponin adjuvant
[0054] Weigh 500 μg of the total saponins of Albizia juniperi, the active active fraction A of Albizia bark saponin adjuvant, or the active effective fraction B of Albizia juniperi saponin adjuvant, and 100 μg of recombinant hepatitis B virus surface antigen (rHBsAg), dissolve in 10 ml of normal saline, and dissolve in 0.22 μm Filtered through a microporous membrane, sterile aliquoted, 1 ml each. Each ml contains saponin 50μg and rHBsAg 10μg.
Embodiment 3
[0055] Example 3 Hepatitis B vaccine preparation containing aluminum gel and saponin adjuvant
[0056] Weigh an appropriate amount of total saponins of Albizia juniperi, the active active part A of Albizia bark saponin adjuvant, or the active effective part B of albizia juniperi saponin adjuvant, dissolve it in normal saline to make a solution with a concentration of 100 μg / ml, and filter it through a 0.22 μm microporous membrane filter. The saponin solution is mixed with an equal volume of aluminum-containing adjuvanted hepatitis B surface antigen vaccine stock solution (each ml contains 20 μg of HBsAg and 500 μg of aluminum hydroxide), mixed evenly, and aseptically dispensed, 1 ml per tube. Each ml contains saponin 50μg, HBsAg 10μg and aluminum hydroxide 250μg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com