Antitumor medicine conjugate with folic acid receptor-mediated and photoresponsive functions, and preparation method thereof
An anti-tumor drug and folic acid receptor technology, which is applied in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems that have not been reported in the literature of photocleavage receptor-targeted anti-tumor drug conjugates, and achieve Toxicity reduction, enhanced effect, extended circulation time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
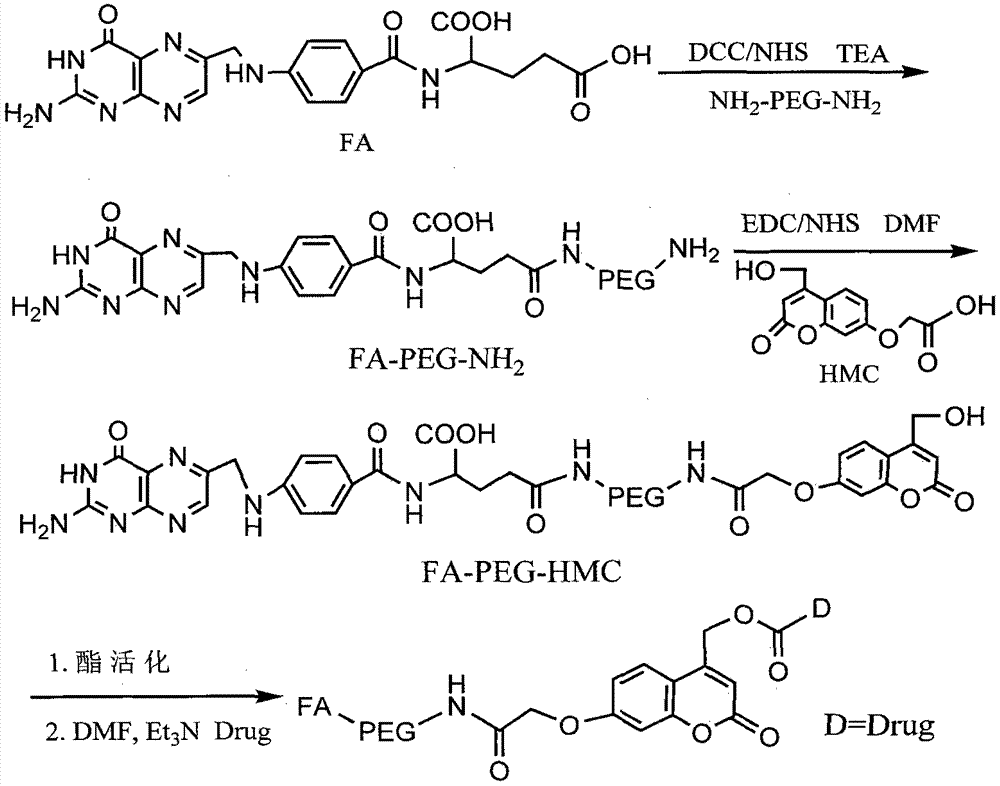
[0029] Preparation of embodiment 1 folic acid-polyethylene glycol-doxorubicin conjugate
[0030] (1) Synthesis of folic acid-polyethylene glycol-photosensitive coupling agent
[0031] 1) Folate-functionalized polyethylene glycol synthesis
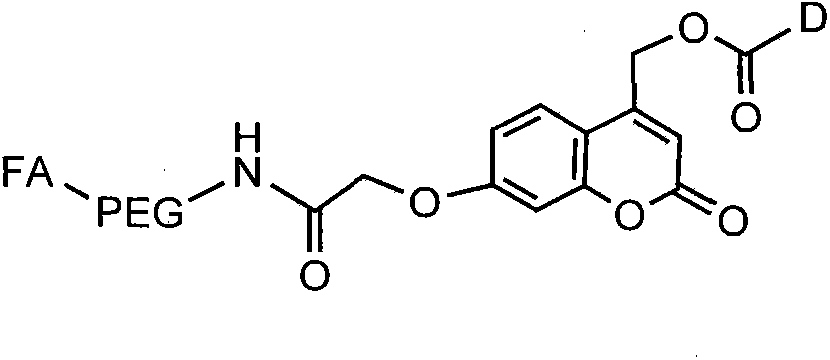
[0032] Weigh double-terminal amino polyethylene glycol (NH 2 -PEG-NH 2 ,M w =3200Da) 640 mg was dissolved in 10 mL of anhydrous DMSO, 2 mL of DMSO solution dissolved with folic acid FA and NHS was added, and then DCC and TEA were added in turn (the molar amount of each substance NH 2 -PEG-NH 2 : FA: NHS: DCC: TEA = 1: 1: 1.5: 1.5: 5), under the protection of nitrogen, stirred at room temperature, protected from light for 12 hours, diluted the reaction mixture with 20 mL of deionized water, and centrifuged at 500 rpm to remove the by-products. The product dicyclohexyl urea, then add 10mL acetone to remove unreacted folic acid, put the supernatant into a dialysis tube with a molecular weight cut-off of 3000Da, dialyze with deionized wate...
Embodiment 2
[0039] Preparation of embodiment 2 folic acid-polyethylene glycol-5-FU conjugate
[0040] (1) According to the step of embodiment 1, the photosensitive group hydroxyl-activated carbonate containing folic acid functionalization is synthesized;
[0041] (2) Preparation of folic acid-polyethylene glycol-5-FU conjugate
[0042] Weigh 150 mg folic acid-functionalized photosensitive group hydroxyl-activated carbonate and dissolve it in 20 mL DMF, add 15 μL TEA dropwise, then add 5 mL DMF solution containing 50 mg 5-FU, stir at room temperature for 12 h, dialyze with DMF for 10 h, and then After dialysis with deionized water for 48 hours, freeze-dried for use.
Embodiment 3
[0043]Example 3 Preparation of Folic Acid-Polyethylene Glycol-Methotrexate Conjugate
[0044] (1) according to the step of embodiment 1 synthetic photosensitive group hydroxyl activated carbonate of folic acid functionalization;
[0045] (2) Preparation of folic acid-polyethylene glycol-methotrexate conjugate
[0046] Weigh 200 mg folic acid-functionalized photosensitive group hydroxyl-activated carbonate and dissolve it in 25 mL DMF, add 20 μL TEA dropwise, then add 5 mL DMF solution in which 50 mg methotrexate is dissolved, stir at room temperature for 12 h, then dialyze with DMF for 10 h, Then it was dialyzed with deionized water for 48 hours, and then freeze-dried for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com