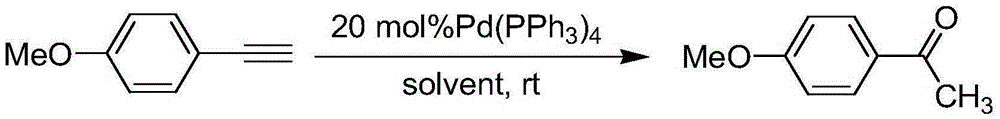A kind of preparation method of p-methoxyacetophenone
A technology of methoxyacetophenone and p-methoxybenzene, which is applied in the field of chemical reagent preparation, can solve problems such as environmental pollution, and achieve the effects of low price, novel design and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
preparation example Construction
[0013] A kind of preparation method of p-methoxyacetophenone proposed by the present invention, add p-methoxyphenylacetylene, tetrakistriphenylphosphine palladium, hydrochloric acid, solvent, the mole of methoxyphenylacetylene and hydrochloric acid successively in the reactor The ratio is 1:1.2, reacted at room temperature for 48 hours, evaporated to dryness, and purified by column chromatography to obtain a light yellow solid product, which is pure p-methoxyacetophenone, and the eluent for column chromatography purification is ethyl acetate The volume ratio of ester to petroleum ether is 1:5.
[0014] In addition, tetrakistriphenylphosphine palladium is a catalyst, and tetrakistriphenylphosphine palladium is 20 mol% of p-methoxyphenylacetylene. The solvent can be selected from anhydrous methanol, anhydrous acetonitrile, and anhydrous dioxane. The reactor is a dry and clean Schlenk bottle, and the Schlenk bottle adopts standard technology, and the whole reaction is carried ou...
Embodiment 1
[0018] Using a dry and clean Schlenk bottle as a reactor, add p-methoxyphenylacetylene (26 μL, 0.2 mmol) and 5 mol / L hydrochloric acid solution (48 μL, 0.24 mmol) under standard Schlenk technique (argon protection), four three Phenylphosphinepalladium (46mg, 0.04mmol) and 3ml of anhydrous methanol. The reaction was stopped after 48 hours at room temperature. The solvent was evaporated to dryness and purified by column chromatography (eluent: ethyl acetate: petroleum ether = 1:5) to obtain pure p-methoxyacetophenone with a yield of 29 mg and a yield of 97%. product by 1 HNMR, 13 Confirmed by CNMR, IR, HRMS. 1 HNMR (400MHz, CDCl 3 )δ2.56(S,3H),3.87(S,3H),6.94(d,J=9.0Hz,2H),7.94(d,J=9.0Hz,2H). 13 CNMR (100MHz, CDCl 3 )δ26.3, 55.4, 113.7, 130.4, 130.6, 163.5, 196.7.
Embodiment 2
[0020] Using a dry and clean Schlenk bottle as a reactor, add p-methoxyphenylacetylene (26 μL, 0.2 mmol) and 5 mol / L hydrochloric acid solution (48 μL, 0.24 mmol) under standard Schlenk technique (argon protection), four three Phenylphosphine palladium (46mg, 0.04mmol) and 3ml of anhydrous acetonitrile. The reaction was stopped after 48 hours at room temperature. The solvent was evaporated to dryness and purified by column chromatography (eluent: ethyl acetate: petroleum ether = 1:5) to obtain pure p-methoxyacetophenone with a yield of 27 mg and a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com