Preparation method of DOTA.2HCl
A technology of formula and dodecane, which is applied in the field of preparation of DOTA·2HCl, can solve the problems of high cost and unsuitability for industrial production, and achieve the effects of low production cost, easy acquisition and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
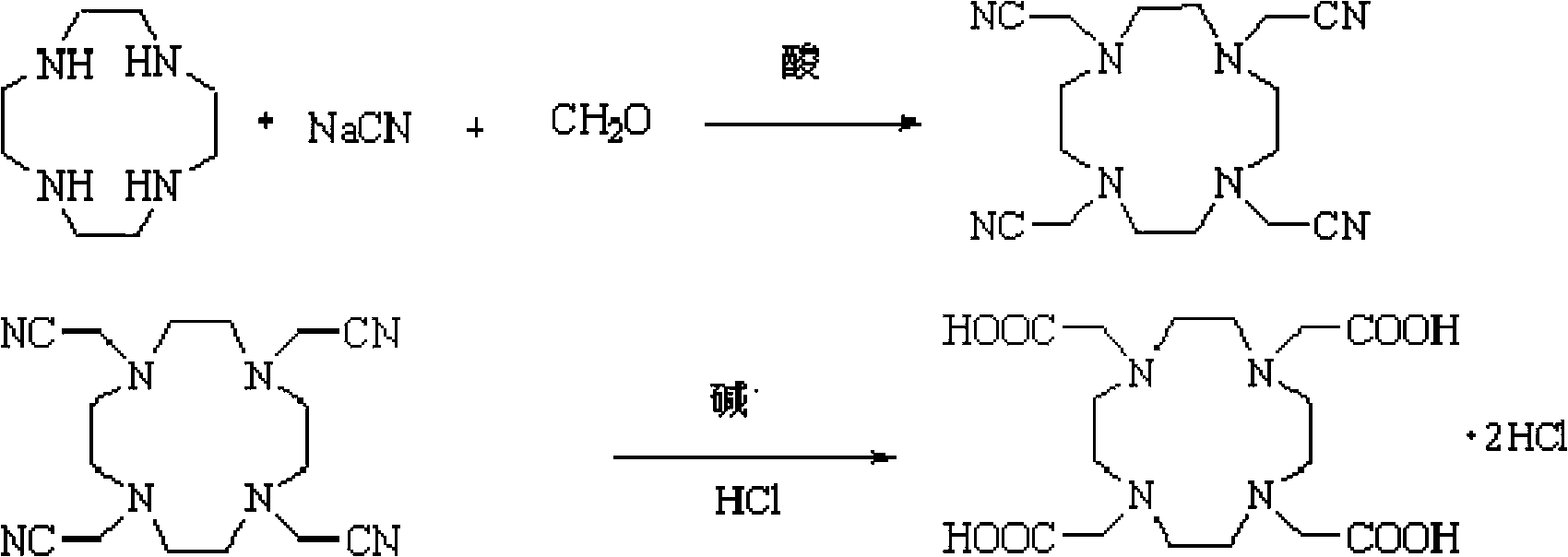
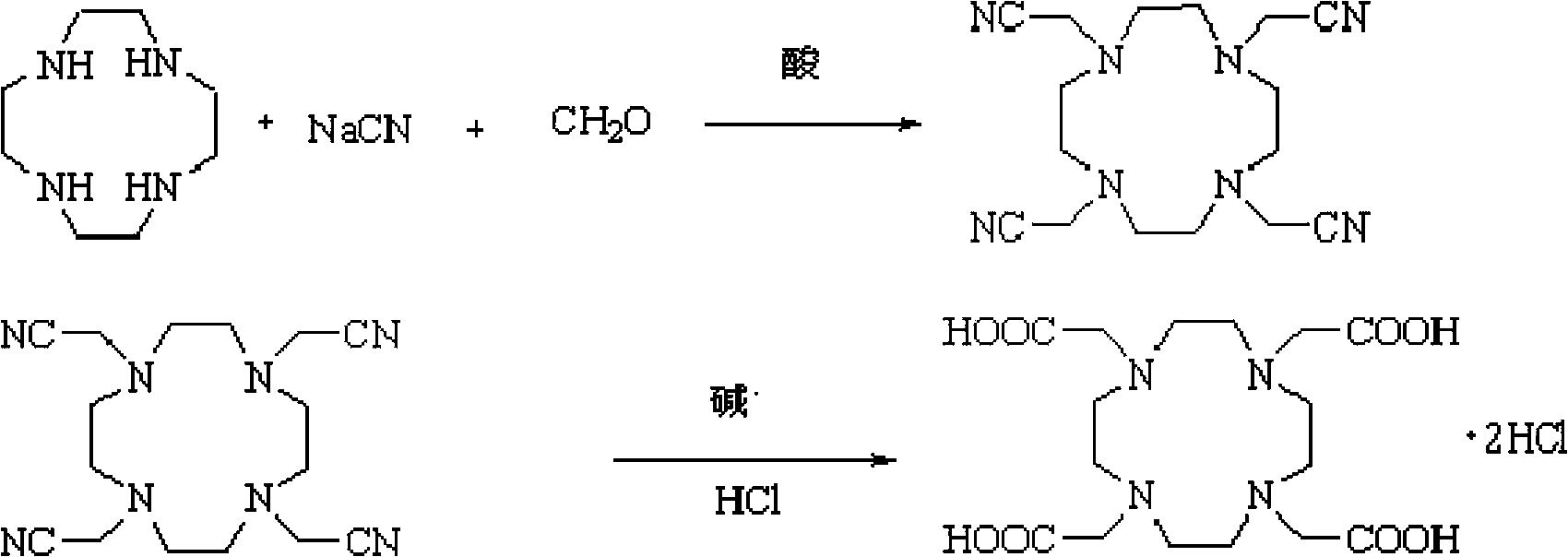
[0019] The preparation method of DOTA.2HCl of the present invention comprises the following steps:
[0020] (1) Preparation of 1,4,7,10-tetrakis(cyanomethylene)-N-tetraazacyclododecane
[0021] Dissolve 1,4,7,10-tetraazacyclododecane (cyclen) in water, adjust the pH to 4-5, cool to 0-3°C, first add a part of formaldehyde solution dropwise, then add formaldehyde and After the mixed solution of acetic acid and sodium cyanide solution, the mixed solution of formaldehyde and acetic acid is dripped, the remaining sodium cyanide solution is continued to be added dropwise, and the acetic acid solution is added dropwise at the same time to maintain the pH between 4.5-6.0. Keep the reaction at ℃ for more than 8 hours, dissolve the filtered solid in water, adjust a small amount of 10% NaOH to pH greater than 10, stir for half an hour, heat the filtered solid with acetone, heat filter to remove inorganic salts, reflux to evaporate half of the acetone, After cooling to allow the product ...
Embodiment 1
[0025] (1) Preparation of 1,4,7,10-tetrakis(cyanomethylene)-N-tetraazacyclododecane
[0026] In a 1000ml four-necked flask, add 250ml of water and 34.4g (0.2mol) of 1,4,7,10-tetraazacyclododecane at one time, and adjust with concentrated hydrochloric acid (about 42ml, 0.4mol) under mechanical stirring. PH = 4-5, use an ice-salt bath to control the reaction solution at 0-3°C, add about 1 equivalent (16g, 0.2mol) of formaldehyde solution (mass fraction 37%) dropwise for about 10 minutes, and the remaining formaldehyde solution 81.3g (1.0 mol) and about 66g (0.9mol) of acetic acid were mixed evenly, and added dropwise at the reaction liquid temperature of 0-3°C, and at the same time, this mixed solution and 196g of sodium cyanide solution (mass fraction was 30%, 1.2mol) were added dropwise for about 4 hours , the acetic acid solution of formaldehyde has been added dropwise. At this time, there is still about 1 equivalent (33g, 0.2mol) of sodium cyanide left. While continuing to a...
Embodiment 2
[0035] (1) Preparation of 1,4,7,10-tetrakis(cyanomethylene)-N-tetraazacyclododecane
[0036]In a 250ml four-necked flask, add 50ml of water and 6.44g (0.04mol) of 1,4,7,10-tetraazacyclododecane at one time, and use concentrated hydrochloric acid (about 8.2ml, 0.08mol) under mechanical stirring Adjust the pH to 4-5, use an ice-salt bath to control the reaction solution at 0-2°C, add about 1 equivalent (3.2g, 0.04mol) of formaldehyde solution (mass fraction 37%) dropwise in about 10 minutes, and the remaining formaldehyde solution is 14.6g (0.18mol) and about 11.8g (0.16mol) of acetic acid were mixed evenly, and added dropwise at the reaction liquid temperature of 0-2°C, and at the same time, this mixed solution and 36.0g of sodium cyanide solution (mass fraction: 30%, 0.22mol) were added dropwise , about 4 hours, the acetic acid solution of formaldehyde was added dropwise, and at this time, about 1 equivalent (6.6g, 0.04mol) of sodium cyanide remained. While continuing to add s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com