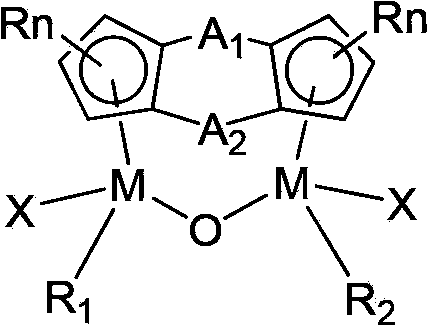
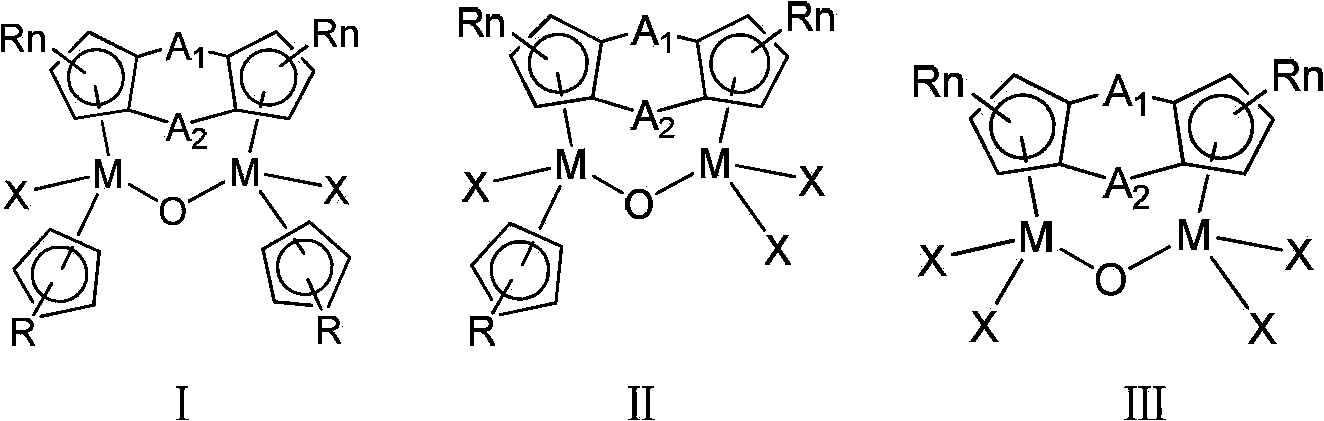
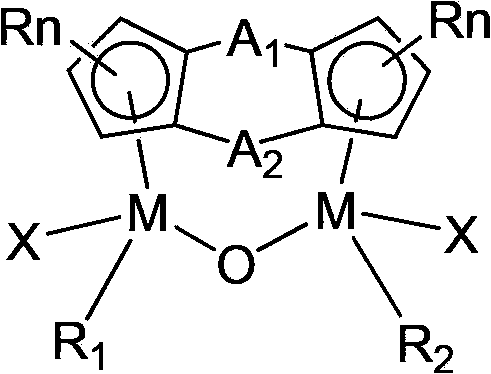
Bridged cyclopentadienyl bimetallic catalyst and use thereof
A technology of cyclopentadiene and cyclopentadienyl is applied in the field of catalysts for preparing broad/bimodal polyethylene, and can solve the problems of poor thermal stability, difficult process control, harsh preparation conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 250ml reaction bottle after evacuated argon displacement, 6.52g (30mmol) of CpTiCl 3 Dissolve in 200ml of THF, then dissolve 0.28g (16mmol) of water into 40ml of THF, and slowly add it dropwise to the above solution. After the dropwise addition, continue to react for 12 hours at room temperature, and then evaporate most of the THF. Then a large amount of n-hexane was added, solids were precipitated, filtered, recrystallized with carbon tetrachloride and dried in vacuo to obtain (CpTiCl 2 ) 2 O4.25g, yield 75%. In a 250ml reaction bottle after vacuum argon displacement, 1.05g (5mmol) of (Me 2 c) 2 (C 5 h 3 ) 2 Dissolve in 50ml n-hexane, slowly add 10mmol n-BuLi hexane solution dropwise at 0°C, after the dropwise addition, slowly rise to room temperature and react for 6 hours to obtain a white suspension, filter the supernatant, drain the solvent, add 60mlTHF, then add 1.91g (5mmol) of (CpTiCl 2 ) 2 O, after stirring at room temperature for 12h, the solvent ...
Embodiment 2
[0024] 0.507g (1mmol) (Me 2 Si)(Me 2 C)(C 5 h 3 ) 2 (CpZrCl) 2 O was dissolved in 30ml THF, and 3ml (12M, 40mmol) concentrated hydrochloric acid was added under cooling in an ice-water bath, then raised to room temperature and continued to react overnight. The solvent was dried under reduced pressure, and the residue was washed with CH 2 Cl 2 Extract, filter, add appropriate amount of n-hexane after the filtrate is concentrated, freeze, filter and precipitate to obtain after vacuum-drying (Me 2 Si)(Me 2 C)(C 5 h 3 ) 2 (CpZrCl)O(ZrCl 2 ) 0.59g, yield 90%. Elemental analysis found values: C, 40.7%; H, 3.9%.
[0025] Add 50ml of toluene distilled out through reflux of metal sodium to the 250ml reactor filled with ethylene after nitrogen replacement three times, stir and heat up to 0°C, feed ethylene to keep the pressure at 2MPa in the reactor, add the main catalyst (Me 2 Si)(Me 2 C)(C 5 h 3 ) 2 (CpZrCl)O(ZrCl 2 ) (the concentration is 9×10 -4 mol / L), co-cataly...
Embodiment 3
[0027] According to the method described in Example 1, 10.5 g (30 mmol) of CpHfCl 3 React with 0.28g (16mmol) of water to get (CpHfCl 2 ) 2 O 6.9 g, yield 72%. Then 1.95g (5mmol) of (Me 2 SiOSiMe 2 ) 2 (C 5 h 3 ) 2 With 4.72g (5mmol) of (CpHfCl 2 ) 2 O reacts to give (Me 2 SiOSiMe 2 ) 2 (C 5 h 3 ) 2 (CpHfCl) 2 O 2.42g, yield 50%. The measured value of elemental analysis: C, 35.0%; H, 4.3%.
[0028] Add 50ml of toluene distilled out through reflux of metal sodium to the 250ml reactor that has been replaced with nitrogen three times and then filled with ethylene, stir and heat up to 90°C, feed ethylene to keep the pressure in the reactor at 3.5MPa, add the main catalyst (Me 2 SiOSiMe 2 ) 2 (C 5 h 3 ) 2 (CpHfCl) 2 O (at a concentration of 2×10 -4mol / L), cocatalyst MMAO (Al / Hf molar ratio is 4000:1), the polymerization time is 1 hour, then add 10ml of acidified ethanol with a concentration of 10% by volume until the reaction is terminated, wash with water ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com