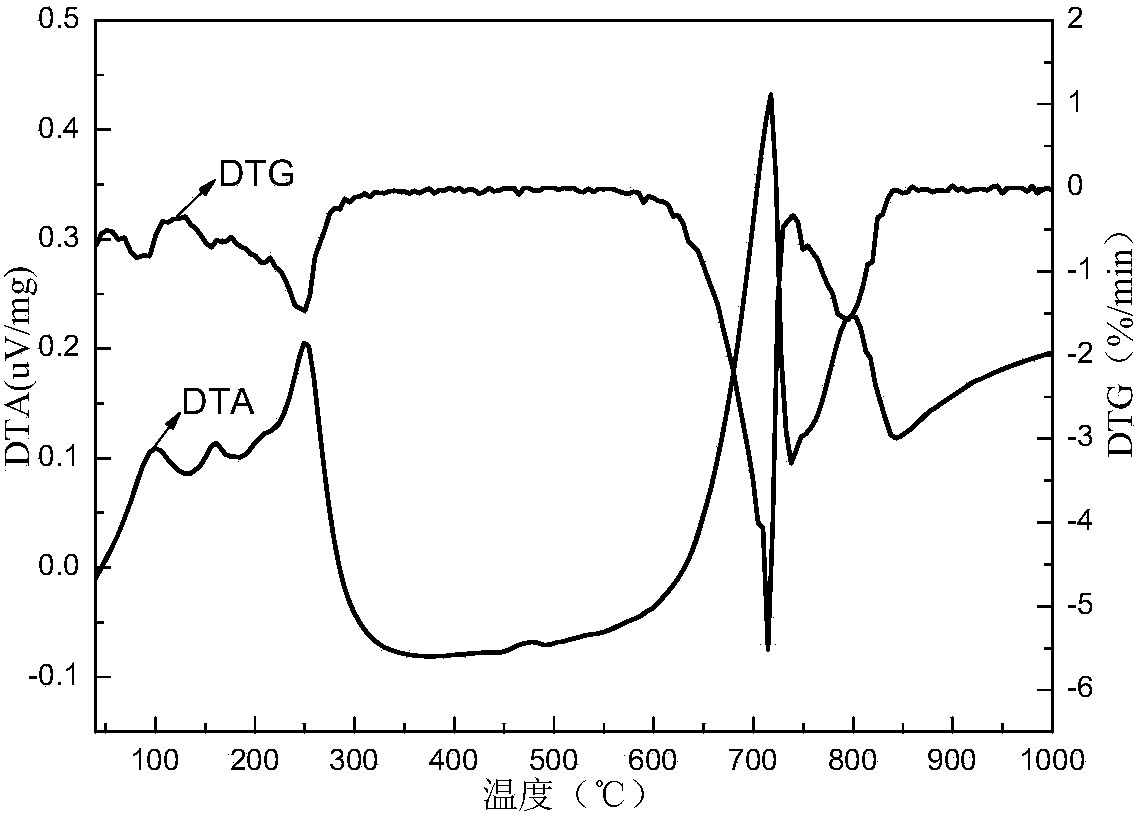
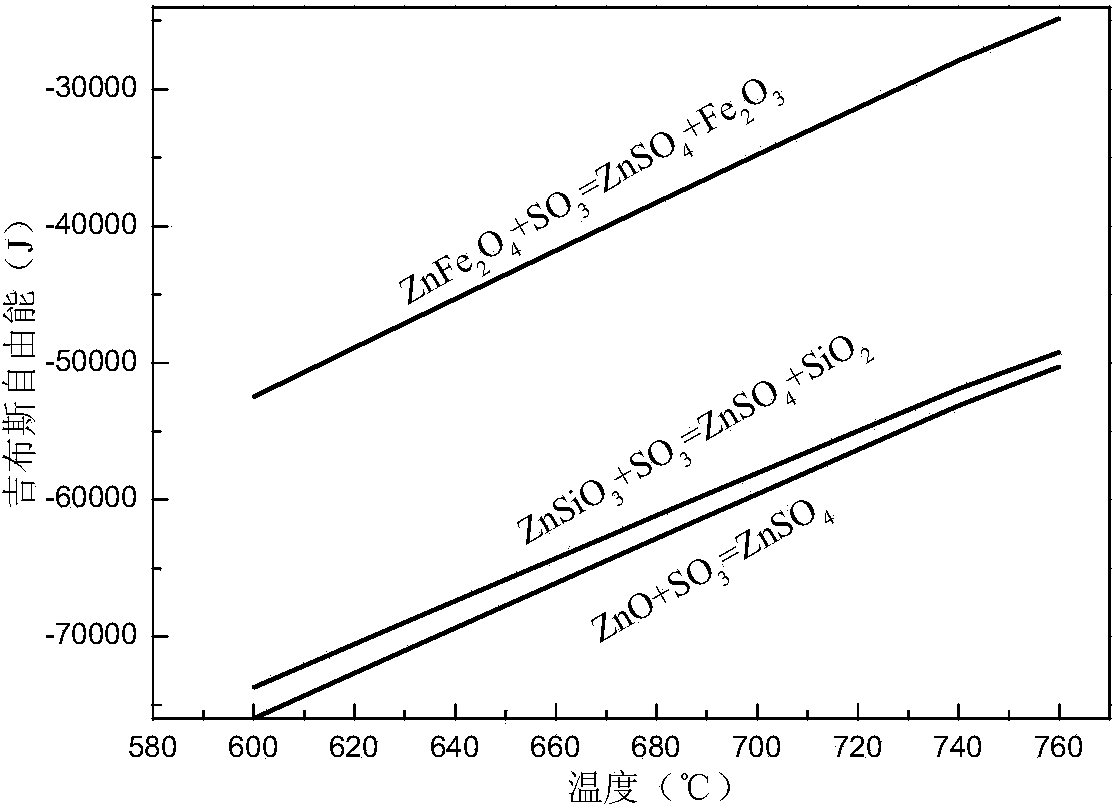
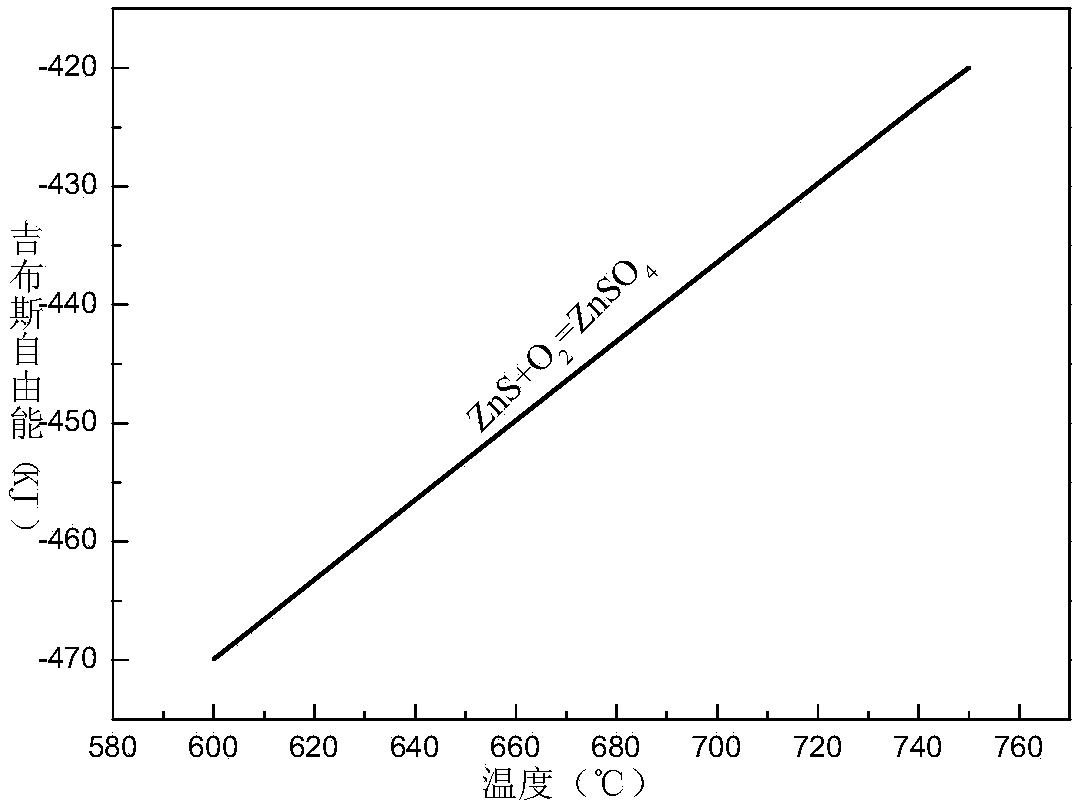
Method for recovering zinc in waste residue containing zinc ferrite through ferric sulfate roasting-water leaching
A technology of ferric sulfate and zinc sulfate, applied in the intersection of metallurgical engineering and environmental engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The mixed slag containing zinc ferrite from a smelter in Hunan is used as raw material, and its chemical composition is shown in the following table:
[0027] Fe
[0028] After uniformly mixing 100g of the above-mentioned mixed slag containing zinc ferrite and 2g-40g of ferric sulfate, roasting at a constant temperature of 600-700°C for 30-90min. The changes in the mass percentage of zinc in various forms and total zinc in the slag before and after roasting are as follows:
[0029] Element
[0030] It can be seen from the above that after the treatment of the present invention, most of the zinc ferrite in the waste residue has been decomposed and converted into zinc sulfate, and the decomposition rate has reached 87.76%. In addition, a small amount of zinc oxide, zinc sulfide, and zinc silicate in the waste residue is also converted into zinc sulfate, so that the mass ratio of zinc sulfate to the total zinc reaches 90.93%. The waste residue treated...
Embodiment 2
[0034] Taking zinc leaching slag from a smelter in Hunan as an example, its chemical composition is as follows:
[0035] Fe
[0036] After uniformly mixing 100 g of the zinc leaching slag containing zinc ferrite with 40-85 g of ferric sulfate, it is roasted at a constant temperature of 600-700° C. for 30-90 min. The changes in the mass percentage of zinc in various forms and total zinc in the slag before and after roasting are as follows:
[0037] Element
[0038] It can be seen from the above that after the treatment of the present invention, most of the zinc ferrite in the waste residue has been decomposed and converted into zinc sulfate, and the decomposition rate has reached 85.54%. In addition, a small amount of zinc oxide, zinc sulfide, and zinc silicate in the waste residue are also converted into zinc sulfate, so that the mass ratio of zinc sulfate to the total zinc reaches 89.08%. The waste residue treated by the present invention is leached with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com