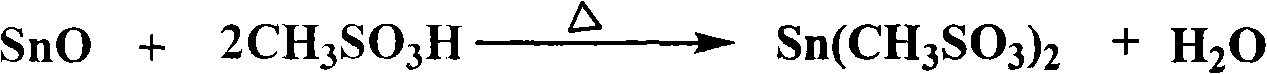Patents
Literature
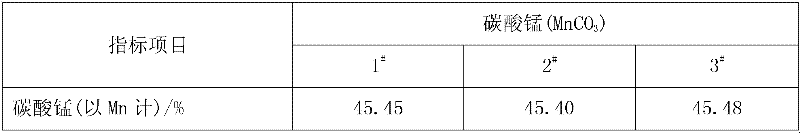
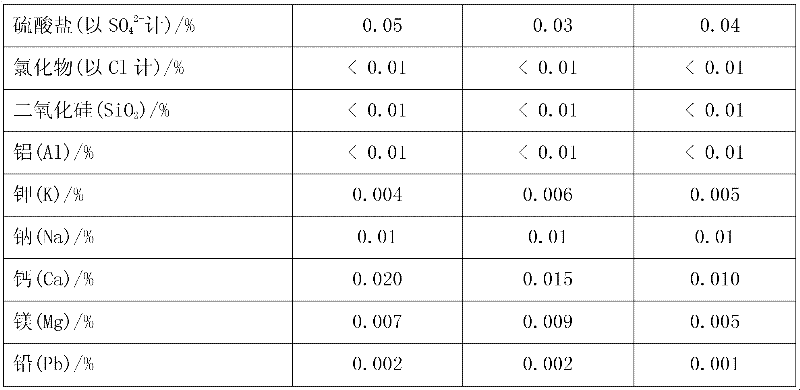
51results about How to "Less impurity ions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
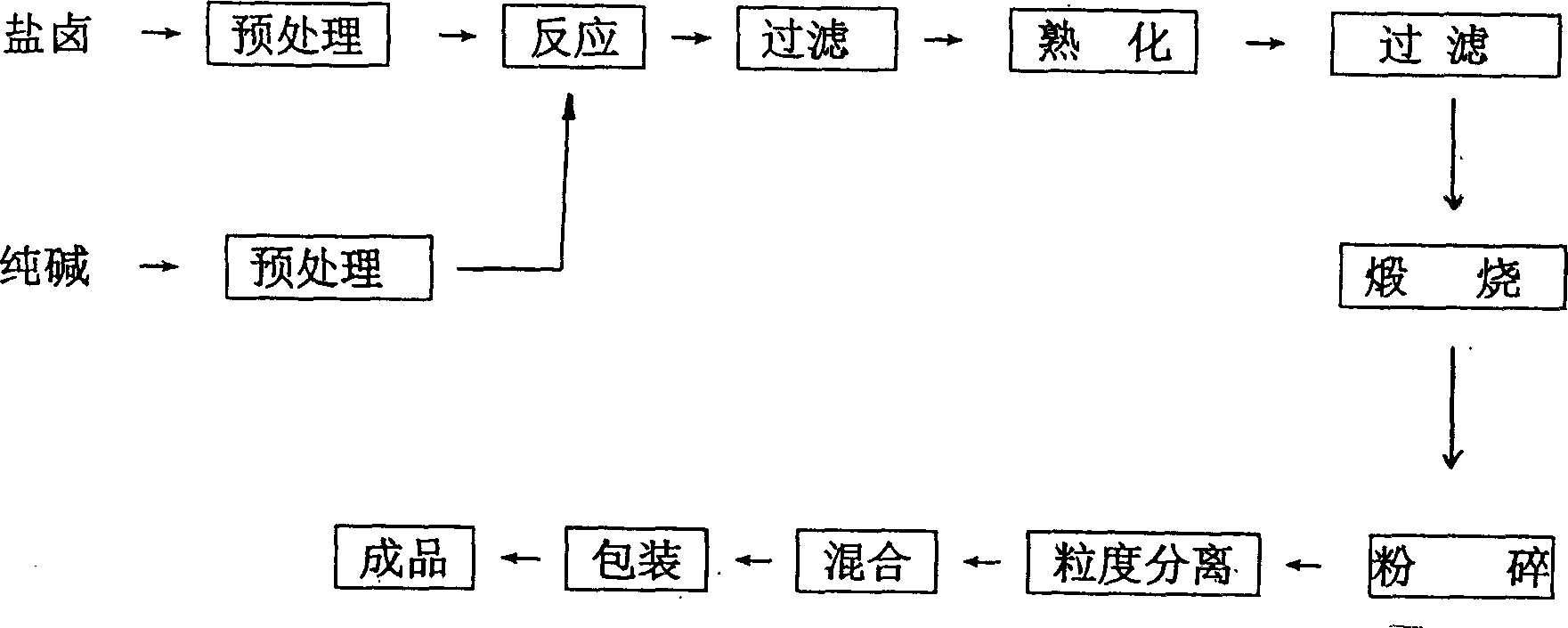
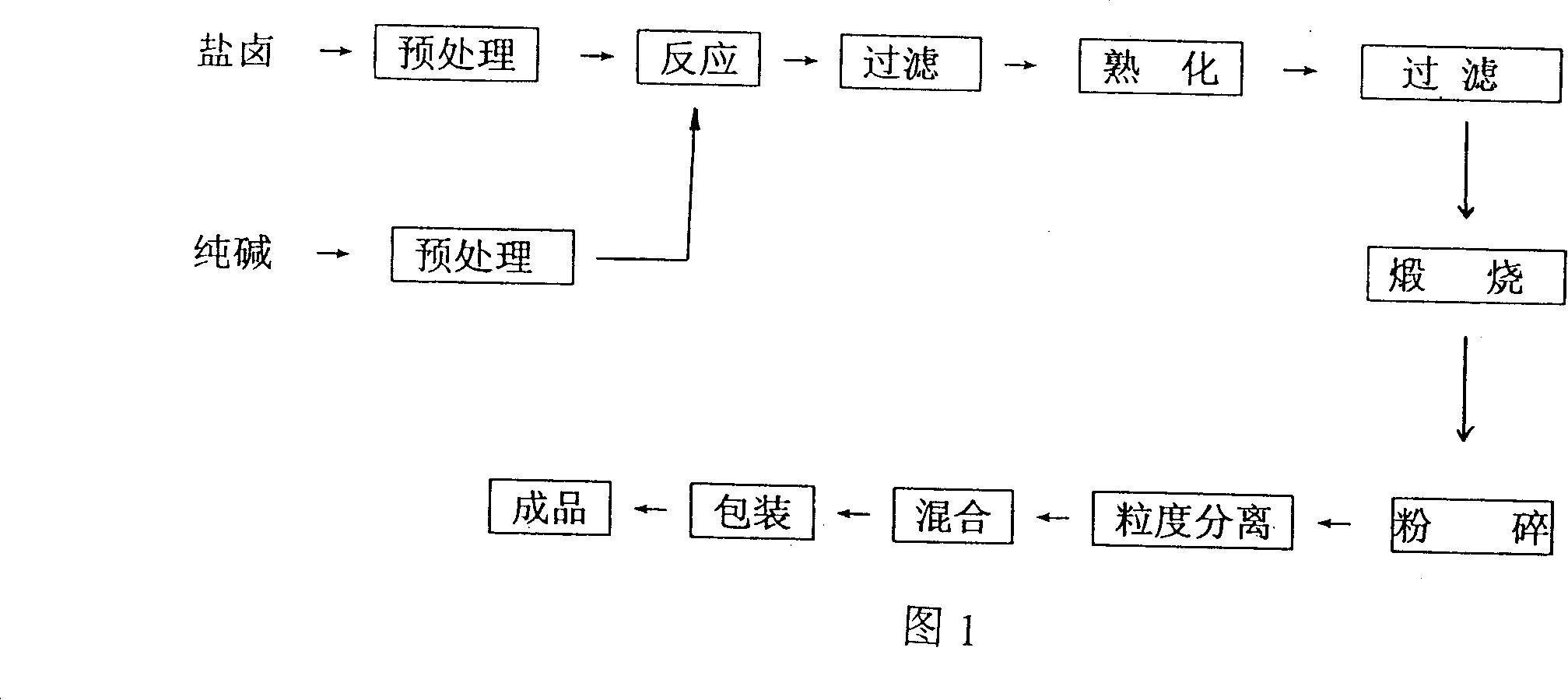
Prepn process of magnesia for silicon steel
The present invention relates to preparation process of magnesium compound, and is especially preparation process of magnesia for silicon steel. The present invention produces silicon steel level magnesia through a bittern-sodium carbonate process, which includes reaction of refined magnesium chloride solution and refined sodium carbonate solution at 65-85 deg.c for 5-15 min to produce basic magnesium carbonate, curing, filtering and calcining at 1100+ / -50 deg.c for 2.0+ / -0.5 hr. Thus produced silicon steel level magnesia has high purity, small average grain size, good coating performance, high adhesivity and other features, and the preparation process is simple and low in cost.
Owner:山西银海锋源新材料有限公司
Method for recovering zinc in waste residue containing zinc ferrite through ferric sulfate roasting-water leaching
ActiveCN103789556AAchieve separationHigh recovery rateProcess efficiency improvementEnvironmental chemistryZinc ferrite
The invention discloses a method for recovering zinc in waste residue containing zinc ferrite through ferric sulfate roasting-water leaching. The waste residue containing zinc ferrite is mixed with a certain amount of ferric sulfate and then obtained mixture is roasted, so that the zinc ferrite is effectively decomposed and converted into zinc sulfate and ferric oxide; at the same time, little zinc, existing in the form of other substances such as zinc oxide, zinc sulfate and zinc silicate, in the waste residue can be converted into zinc sulfate under the condition, and is leached in a following washing process; the ferric oxide enters into leached slag, thus realizing zinc and iron separation; leachate, which is a zinc sulfate solution containing few impurity irons, can be directly returned to a hydrometallurgical process to recover zinc. The process disclosed by the invention is simple, environmental friendly and energy-saving, and the process effectively solves problems that a conventional wet hydrometallurgical process which is difficultly used to separate zinc and iron, is not high in zinc recovery rate, serious in environmental pollution and the like.
Owner:CENT SOUTH UNIV
Gas-sensor nanometer sensitive material, slurry with gas-sensor nanometer sensitive material, preparing method of gas-sensor nanometer sensitive material, preparing method of slurry and application of gas-sensor nanometer sensitive material
InactiveCN105259211AEasy to prepareHigh yieldMaterial nanotechnologyMaterial analysis by electric/magnetic meansDispersitySlurry
The invention relates to a gas-sensor nanometer sensitive material, slurry with the gas-sensor nanometer sensitive material, preparing of the gas-sensor nanometer sensitive material, preparing of the slurry and an application of the gas-sensor nanometer sensitive material. A preparing method of the gas-sensor nanometer sensitive material includes the following steps that 1, stannate is added into ultrapure water and subjected to ultrasonic dispersion, a stannate solution is obtained, a urea ethanol solution is added, ultrasonic processing continues, an obtained mixed solution is transferred into a hydrothermal reaction kettle, after reaction is completed, cooling is carried out, and bottom precipitate is collected, centrifugally washed, arranged in a dryer and dried; 2, nanometer SnO2 hollow sphere powder obtained after drying is added into distilled water to be subjected to ultrasonic dispersion, a Pd(NO3)2 solution is dropwise added under the stirring condition, ammonium hydroxide is added till the pH of the mixed solution ranges from 9 to 12, the mixture is stirred at the indoor temperature, bottom precipitate is centrifugally collected, washed to be neutral and dried, and finally Pd-doping nanometer SnO2 hollow spheres are obtained. The gas-sensor nanometer sensitive material, the slurry, the preparing and the application have the advantages that the preparing methods are simple, the quantity of introduced foreign ions is small, the yield is high, volume production is facilitated, the specific area of the material is large, dispersity is good, and high sensitivity and short response recovery time are achieved.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Method for producing water-soluble potassium ammonium phosphate from wet-process phosphoric acid
ActiveCN103011122AHigh purityLess impurity ionsSilicon halogen compoundsPhosphorus compoundsPhosphatePhosphoric acid
The invention relates to a method for producing water-soluble potassium ammonium phosphate from wet-process phosphoric acid. According to the method, a monoammonium phosphate solution is prepared from low-cost wet-process phosphoric acid as the raw material by the steps of desulfuration and defluorination and neutralization with ammonia; then the replacement reaction of the monoammonium phosphate solution and potassium chloride and the crystallization are carried out to obtain a water-soluble fertilizer which contains potassium dihydrogen phosphate as a main component and adopts ammonium dihydrogen phosphate as an auxiliary component, wherein the content of chlorine is smaller than 2%; and excess potassium chloride and nitrogen produce a by-product such as nitrogen phosphorus and potassium chloride water soluble compound fertilizer. Meanwhile, the by-product in the production process can be directly used as a citric acid soluble phosphatic fertilizer. The method has simple processes, and the prepared water-soluble ammonium potassium dihydrogen phosphate is high in purity, nutrient and potassium content. No waste residues are discharged in the whole process, so that the method is environment-friendly and has better social and economical benefits.
Owner:KINGENTA ECOLOGICAL ENG GRP
Method for preparing iron p-toluenesulfonate and solution thereof
ActiveCN102911089AFerric hydroxide is activeHigh puritySulfonic acids salts preparationReaction intermediateP-toluenesulfonate
The invention discloses a method for preparing iron p-toluenesulfonate and a solution thereof. The method for preparing the iron p-toluenesulfonate includes the steps that (1) a sodium hydroxide solution and an ammonium ferric sulfate solution are prepared; (2) the sodium hydroxide solution is added in the ammonium ferric sulfate solution, the final potential of hydrogen (pH) value of reaction is 7.0 to 9.0, and iron hydroxide precipitation is obtained; (3) the iron hydroxide precipitation is washed, placed in a filter bag and suspended to be dried at room temperature until the iron hydroxide precipitation which is colloid and contains a small amount of water is formed; (4) iron hydroxide which is suspended to be dried is reacted with a p-toluene sulfonic acid to generate an iron p-toluenesulfonate solution; (5) the iron p-toluenesulfonate solution is filtered and concentrated to be viscous, and discharging-cooling and crystallization are performed; and (6) crystals are crushed and dried to obtain the product. The method for preparing the iron p-toluenesulfonate and the solution thereof has the advantages that the activity of the reaction intermediate iron hydroxide is good, the reaction with the p-toluene sulfonic acid is complete, the process is simple, the energy consumption and the cost are low, and the environmental pollution is small.
Owner:广州化学试剂厂
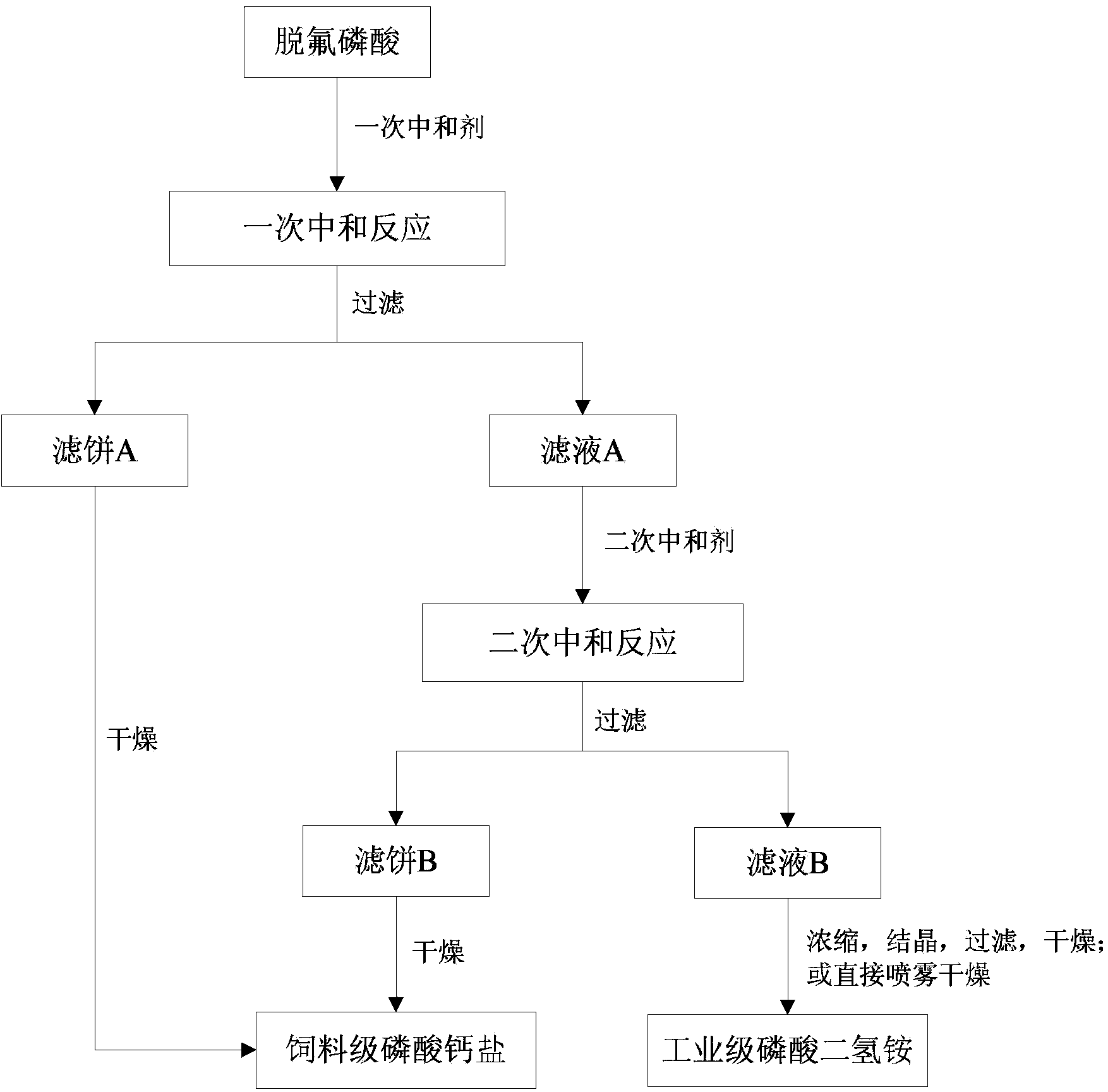
Method for co-production of industrial grade ammonium dihydrogen phosphate and feed grade calcium phosphate by using defluorinated phosphoric acid
The invention relates to a method for co-production of industrial grade ammonium dihydrogen phosphate and feed grade calcium phosphate by using defluorinated phosphoric acid, belonging to the technical field of chemical industry production. The invention aims at solving a technical problem of providing a method for co-production of industrial grade ammonium dihydrogen phosphate and feed grade calcium phosphate by using defluorinated phosphoric acid. The method comprises the following steps: neutralizing the defluorinated phosphoric acid and a primary neutralizer, performing solid-liquid separation to obtain a filtrate A and a filter cake A, neutralizing the filtrate A and a secondary neutralizer to make pH of reaction liquid reach 3.0-5.0, and performing solid-liquid separation to obtain a filtrate B and a filter cake B; concentrating, crystallizing, filtering and drying the filtrate B; or directly performing spray drying at 60-200 DEG C on the filtrate B to obtain the industrial grade ammonium dihydrogen phosphate; and drying the filter cake A and the filter cake B in combination to obtain the feed grade calcium phosphate. According to the method provided by the invention, the technology is simple, generation of waste residue is avoided, raw materials are fully used, and purity of the produced products is high, so that the method has better social benefit and economic benefit.
Owner:SINOCHEM YUNLONG +1
Production method of ore brine crystal salt
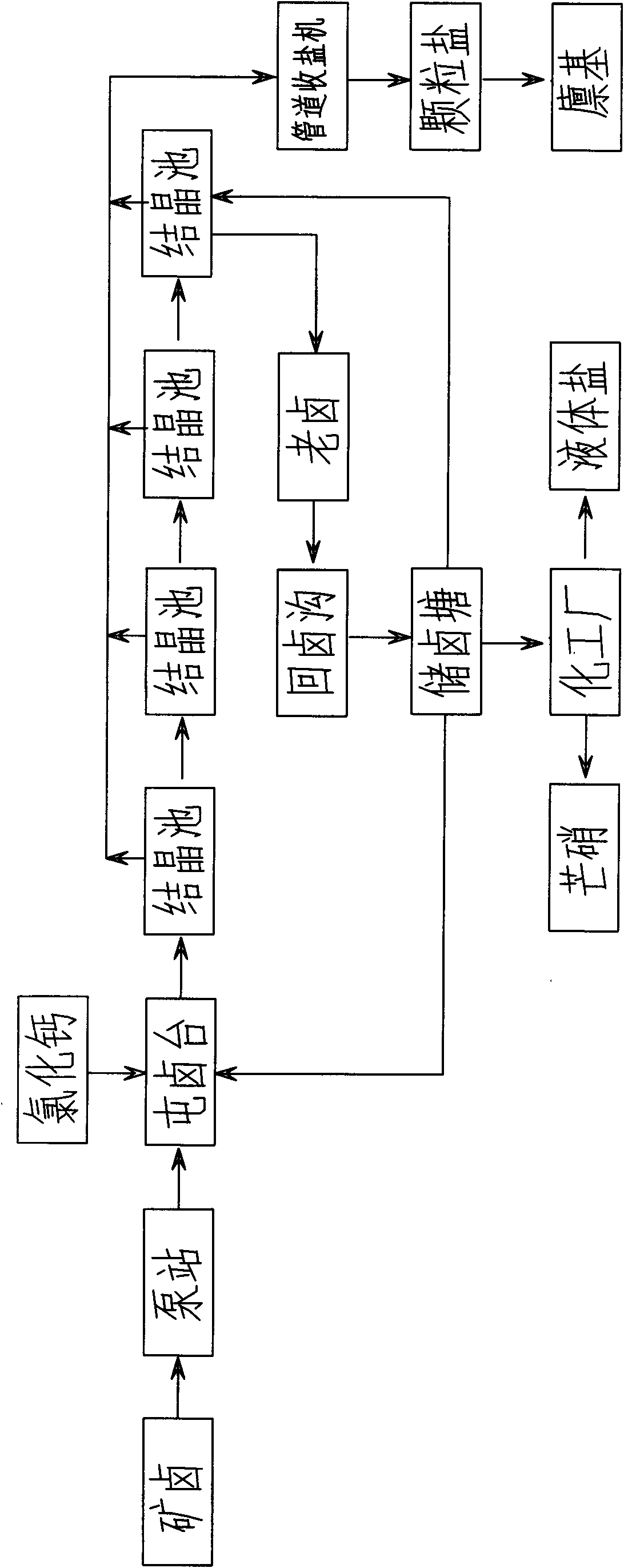
InactiveCN102205977AReduce energy consumptionLarge crystal particlesAlkali metal chloridesAlkali metal sulfites/sulfatesImpurity ionsSea salt
The invention provides a production method of an ore brine crystal salt. In the method, ore brine is used as raw brine, wind and sunlight are used as energy sources, and a shoal pool is used as a crystallization pool. The production method is characterized by comprising the following steps: after exploiting the ore brine, firstly passing the exploited ore brine through a pipeline and then weighing with a pump station, respectively storing the weighed ore brine crystallization salt in a brine hoarding table, pouring to a crystallization pool from the brine hoarding table, and evaporating and crystallizing through the wind and sunlight in the crystallization pool; and controlling the concentration and depth of the brine, properly adding or replacing novel brine, mastering the depth and market demand of the crystal salt, discharging the brine and recovering the salt timely, and recycling the old brine. According to the invention, energy consumption is low, 0.18 ton of coal and 50-60kw.h of electricity are consumed for producing every ton of ore salt, and the electricity consumption of every ton of salt is about 5kw.h in the invention; large land area is saved and accounts for 1 / 20 of that of sea salt; the method is environmentally-friendly, and zero emission is basically achieved; and the produced ore brine crystal salt has high quality, meets a GB / T5 462-2003 prior industrial salt standard, has extremely less impurity ions and large crystalline particles, and is especially suitable for a chlor-alkali industry.
Owner:江苏省东泰盐业投资管理有限公司
High-purity iron p-toluenesulfonate solution synthesis method
InactiveCN105017091AFerric hydroxide is activeHigh puritySulfonic acids salts preparationReaction intermediateImpurity ions
The present invention belongs to the technical field of electronic chemical production, and particularly relates to a high-purity iron p-toluenesulfonate solution synthesis method, which comprises: 1) preparing a ferric sulfate solution and a sodium hydroxide solution; 2) preparing ferric hydroxide floc; 3) preparing ferric hydroxide filter cake having sulfate ions achieving the standard; 4) preparing a p-toluenesulfonic acid solution having qualified sulfate ions; and 5) preparing the high-purity iron p-toluenesulfonate solution. According to the present invention, the prepared reaction intermediate ferric hydroxide has good activity and completely reacts with p-toluenesulfonic acid, the product has high purity, and the impurity ions are less.
Owner:武汉海斯普林科技发展有限公司
Method for preparing electronic-grade high-purity stannous methanesulfonate solution
The invention discloses a method for preparing an electronic-grade high-purity stannous methanesulfonate solution. The method comprises the following steps: (1) placing methanesulfonic acid and stannous oxide with the molar ratio thereof being 3:1-6:1 in a container, and heating up to 100-130 DEG C, reacting for 4-6h while stirring until the stannous oxide completely dissolves, so as to obtain a reaction liquid; (2) crystallizing the reaction liquid by cooling, filtering to obtain a filter cake, and washing and vacuum-drying the filter cake to obtain white stannous methanesulfonate crystals; and (3) adding stannous methanesulfonate to de-ionized water to obtain the stannous methanesulfonate solution. The method provided by the invention for preparing the electronic-grade high-purity stannous methanesulfonate solution has fewer impurity ions; containing only stannous methanesulfonate and water, the prepared product can be easily separated, thereby ensuring the high purity of stannous methanesulfonate solids; and the invention has the advantages of simple process, low cost and less environmental pollution.
Owner:广东光华化学厂有限公司
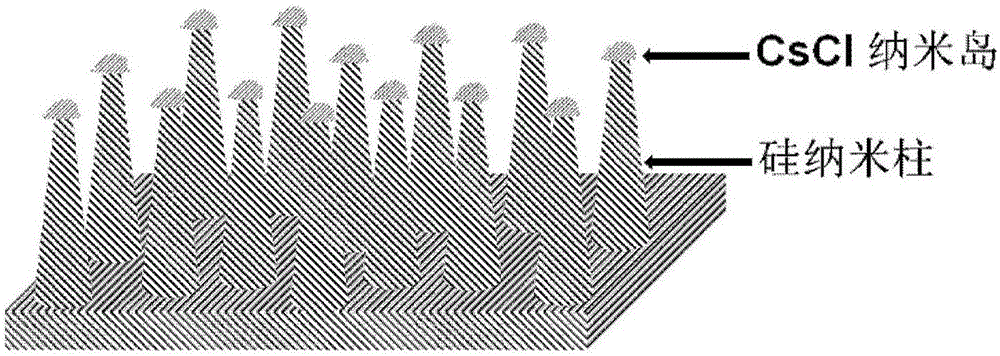
Heterojunction solar cell taking silicon nanorod array as substrate and fabrication method of heterojunction solar cell
InactiveCN106206779AImprove trapping effectIncrease surface ratioFinal product manufacturePhotovoltaic energy generationHeterojunctionTrapping
The invention discloses a heterojunction solar cell taking a silicon nanorod array as a substrate and a fabrication method of the heterojunction solar cell. The solar cell uses a silicon nanorod array with a large depth-width ratio as the substrate, and other thin film materials are wrapped by a magnetron sputtering method to form a heterojunction structure. The fabrication method comprises the following steps of fabricating the silicon nanorod array with the large depth-width ratio on a surface of a P-type silicon wafer by a self-assembly method of a cesium chloride nanometer island; fabricating an aluminum back filed on a back surface; wrapping a surface of the silicon nanorod array with an N-type material layer such as zinc oxide and cadmium sulfide by the magnetron sputtering method; covering a surface of the N-type material layer with an ITO transparent conductive layer; and fabricating a titanium-silver electrode on an upper surface. The solar cell taking the silicon nanorod array with the large depth-width ratio as the substrate has the advantages that 1, the surface ratio of the substrate can be effectively increased, the effective area of a heterojunction is expanded, and absorption to incident light is improved; and 2, by means of a favorable light trapping effect, reflection can be reduced, and the performance of the heterojunction cell is improved.
Owner:INST OF HIGH ENERGY PHYSICS CHINESE ACAD OF SCI
Method for efficiently removing fluorine ions in manganese sulfate solution
InactiveCN108118152AWith physical adsorptionEasy to separateProcess efficiency improvementIon contentSulfate
The invention provides a method for efficiently removing fluorine ions in a manganese sulfate solution. The method is characterized in that a certain quantity of to-be-processed manganese sulfate solution is added into reaction equipment, the content of the fluorine ions in the solution is measured, solid cerium hydroxide with a certain fluorine ion content proportion is added under the conditionsthat the temperature is 30-50 DEG C and the pH value is 2-6, the material is stirred for 8-12 h at the rotational speed of 150-350 r / min, then stirring is stopped, the material is left to stand for 12-18 h and then transferred into filtering equipment for filtration, the filtrate is a fluorine-removed qualified manganese sulfate solution, filter cake is added into reverse osmosis water at a solid-liquid mass ratio of 1:(1-6), stirring and washing are performed for 0.5 h, and filtering is performed to obtain fluorine-containing cerium hydroxide which is then fed into a regeneration procedure.According to the method, lots of residual fluorine ions in a manganese sulfate solution system can be removed, the content of a fluoride in the processed solution can be reduced to 100 ppm or below, and the fluorine removal agent cerium hydroxide can be recycled after being regenerated, so that the production cost is greatly lowered.
Owner:GUANGDONG GUANGHUA SCI TECH
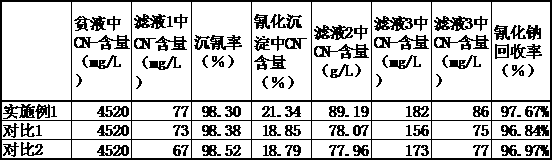
Method for environmentally-friendly treatment and recycling of cyanide barren solution
ActiveCN109385538AAvoid it happening againReduce difficultyProcess efficiency improvementCopperSodium cyanide
The invention relates to a method for environmentally-friendly treatment and recycling of a cyanide barren solution. The method comprises the following steps: carrying out cyanide sedimentation operation on a cyanide-containing barren solution, carrying out cyaniding precipitate conversion, acidifying carbonic acid residue filter cakes, preparing a cyanide sedimentation agent, and feeding back obtained filtrate to a cyanide leaching procedure to achieve the environmentally-friendly treatment and recycling of the cyanide barren solution. By adopting the method provided by the invention, the environmentally-friendly treatment and recycling of the cyanide barren solution are achieved, a process of direct barren solution acidification is avoided, sodium cyanide is in liquid, and thus environment pollution caused by hydrogen cyanide is greatly reduced; the sodium cyanide is recycled and reused with copper and zinc comprehensively, the production cost is lowered, and environment protection requirements on treatment of the cyanide-containing barren solution can be met.
Owner:刘西分
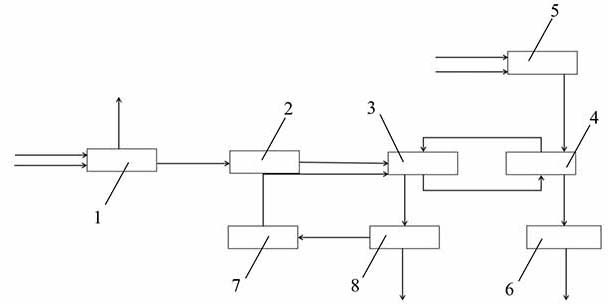
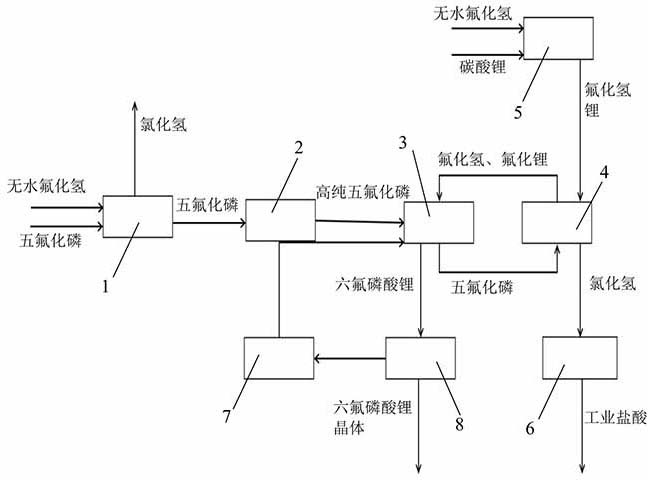
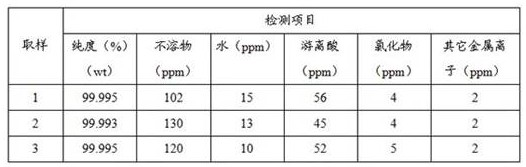
Production device and production method of lithium hexafluorophosphate
ActiveCN114602406AIncrease profitImprove responseDistillation separationLithium hexafluorophosphateLithium carbonatePhosphoric acid
Owner:BEIJING UNIV OF CHEM TECH
Water-based metal rust inhibitor
The invention discloses an aqueous metal anti-rust agent containing cyclohexylamine citrate. The aqueous metal anti-rust agent has good anti-rust effects on iron, steel, iron alloys, aluminum, copper and the like, and thus can be utilized for rust protection of metal equipment and parts, wherein the metal equipment and parts are made of iron, steel, iron alloys, aluminum, copper and the like.
Owner:广州化学试剂厂
Preparation method of silicon-steel-level magnesium oxide coating
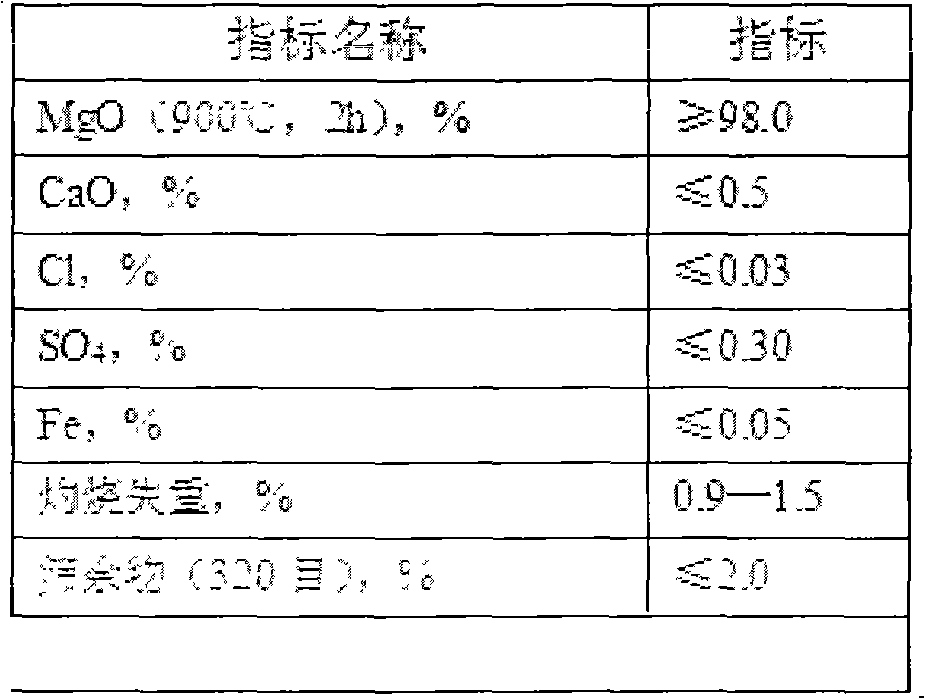
The invention discloses a preparation method of silicon-steel-level magnesium oxide coating especially for oriented silicon steel plates. The preparation method comprises selecting natural aphanitic magnesite to produce lightly-calcined magnesia powder; digesting the lightly-calcined magnesia, performing acid-base neutralization to remove calcium, reacting for 5-15 minutes at 15-30 DEG C to generate magnesium hydroxide, washing with water, filtering, drying, calcining for 2.0 + / - 0.5 hours under a temperature of 1100 + / - 50 DEG C to obtain the high-purity lightly-calcined magnesia powder; and processing the powder into desired micron-level particle sizes by using a jet mill. The silicon-steel-level magnesium oxide coating provided by the invention has characteristics of high chemical purity, low impurity ions, good suspension, low hydration rate, and good coating and adhesion, has physical properties far better than those of similar products, is a good annealing isolation agent for producing the oriented silicon steel plates and also simple in production operation, and can greatly reduce production cost.
Owner:抚顺博达节能环保科技有限公司
Internally circulating electrolysis type ozone generation device
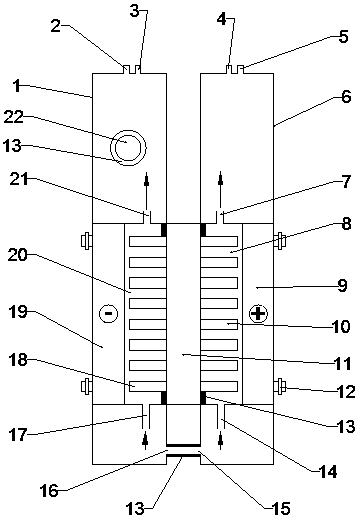
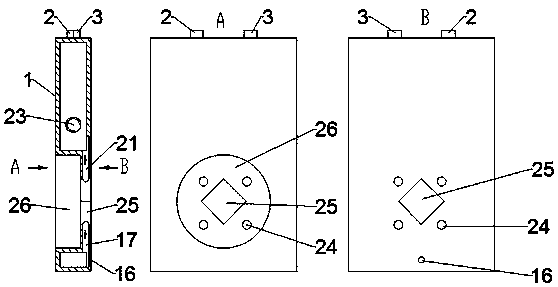
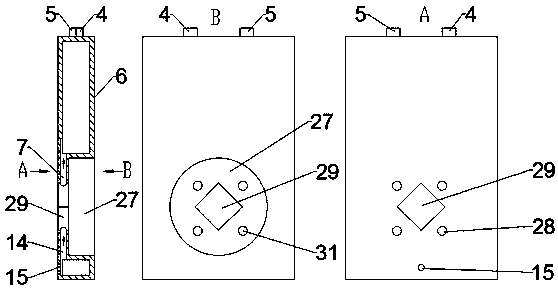
The invention belongs to the technical field of electrochemistry, and relates to an internally circulating electrolysis type ozone generation device. A cavity is formed in the lower portion of a cathode water tank, the interior of the cavity is used as a cathode chamber for reacting, and a circulating channel with raw material water is formed between the cavity and the cathode water tank, so thatthe cavity and the cathode water tank form an integrated cathode reaction assembly. In the same way, a cavity is formed in the lower portion of an anode water tank, the interior of the cavity is usedas an anode chamber for reacting, and a circulating channel with raw material water is formed between the cavity and the anode water tank, so that the cavity and the anode water tank form an integrated anode reaction assembly. An ion exchange resin column is arranged on the upper portion of the anode water tank component. The device is convenient to assemble, compact in structure and good in heatdissipation effect, the ozone concentration is high, and running is stable.
Owner:WUHAN WEIMENG ENVIRONMENTAL TECH
Preparation method for nanoscale lithium iron phosphate
InactiveCN103715428ALess impurity ionsHigh purityCell electrodesSecondary cellsFerric hydroxideLithium iron phosphate
The invention relates to a method for synthesizing a nanoscale lithium iron phosphate positive electrode material, and specifically relates to a method for synthesizing a lithium iron phosphate material in a liquid phase by using synthetic iron hydroxide as a reactant. The method can reduce impurity ions in the material and reduce particle size. Besides, the performance of the material is comprehensively increased by introducing liquid phase C coating.
Owner:BEIJING EASPRING MATERIAL TECH CO LTD
Method for preparing high-purity manganese sulfate and high-purity manganese carbonate by reduction leaching of pyrolusite through scrap iron
InactiveCN102070198BReasonable useSimple processAmmonium sulfatesIron sulfatesVulcanizationPyrolusite
The invention discloses a method for preparing high-purity manganese sulfate and high-purity manganese carbonate by reduction leaching of pyrolusite through scrap iron. The method comprises the following steps of: performing reduction leaching on the pyrolusite with the scrap iron in a sulfuric acid medium to obtain manganese, regulating the pH value to be 5-6 by using a neutralization method, hydrolyzing ferric iron in the solution into an iron hydroxide precipitate, and filtering to obtain primary filtrate of manganese sulfate and filter residue; adding a vulcanizing agent into the primary filtrate for vulcanization to remove heavy metal ions, adding a fluorinating agent for removing calcium, magnesium and other ions in the solution, standing the solution and removing sediments; and concentrating and crystallizing the purified solution to prepare the high-purity manganese sulfate, or adding ammonium carbonate to prepare high-purity manganese carbonate, concentrating the filtrate andrecovering the ammonium carbonate, wherein the filter residue in the first step is leached by solution of dilute sulfuric acid, and the filtrate after filtration is subjected to calcium removal, standing and concentration to form polyferric sulfate. The method is low in cost and investment, is suitable for comprehensive exploitation and utilization of different grades of pyrolusite, can effectively utilize natural resources and also can create obvious economic benefit.
Owner:HUNAN UNIV OF SCI & TECH
Method for producing water-soluble potassium ammonium phosphate from wet-process phosphoric acid
ActiveCN103011122BHigh purityLess impurity ionsSilicon halogen compoundsPhosphorus compoundsO-Phosphoric AcidAgricultural engineering
The invention relates to a method for producing water-soluble potassium ammonium phosphate from wet-process phosphoric acid. According to the method, a monoammonium phosphate solution is prepared from low-cost wet-process phosphoric acid as the raw material by the steps of desulfuration and defluorination and neutralization with ammonia; then the replacement reaction of the monoammonium phosphate solution and potassium chloride and the crystallization are carried out to obtain a water-soluble fertilizer which contains potassium dihydrogen phosphate as a main component and adopts ammonium dihydrogen phosphate as an auxiliary component, wherein the content of chlorine is smaller than 2%; and excess potassium chloride and nitrogen produce a by-product such as nitrogen phosphorus and potassium chloride water soluble compound fertilizer. Meanwhile, the by-product in the production process can be directly used as a citric acid soluble phosphatic fertilizer. The method has simple processes, and the prepared water-soluble ammonium potassium dihydrogen phosphate is high in purity, nutrient and potassium content. No waste residues are discharged in the whole process, so that the method is environment-friendly and has better social and economical benefits.
Owner:KINGENTA ECOLOGICAL ENG GRP
Method for preparing stannous sulfamate solution and application thereof
Owner:GUANGDONG GUANGSHI REAGENTS TECH
Method for preparing stannous sulfamate solution and application thereof
This invention discloses a method for preparing stannous sulfamate solution and an application thereof. The method comprises the following steps of: (1) taking stannous oxide, and adding pure water for rinsing; (2) mixing sulfamic acid and cleaned stannous oxide, and adding pure water, reducing agent and tartaric acid; heating the mixture to 60-90 DEG C, agitating and reacting for 2-4 hours to obtain solution A; (3) adding active carbon to the solution A, absorbing for 0.5-1.5 hours before filtering, and taking filtrate; (4) testing the content of stannous sulfamate in the filtrate and addingpure water to prepare the tin sulfamate solution with the needed concentration. The stannous sulfamate solution prepared by the method is high in purity and less in impurity ions; the preparation method provided by the invention has the advantages of simple process, less energy consumption, low cost and small pollution on the environment; and the method overcomes the disadvantages of the traditional method as follows: the operation cycle is long, the impurity ions are many and are difficult to treat, and the environment is polluted.
Owner:GUANGDONG GUANGSHI REAGENTS TECH
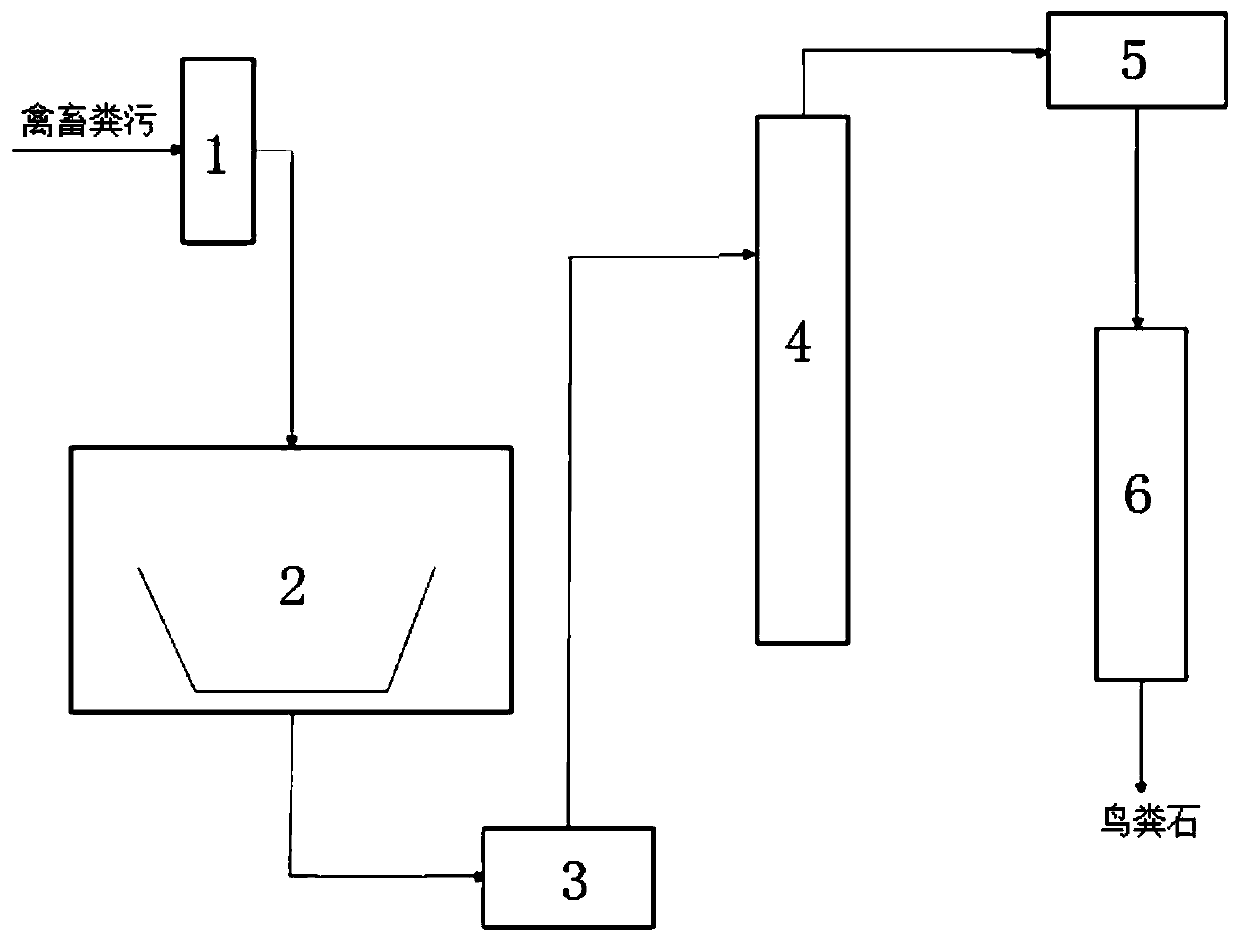
Method for recovering and purifying struvite from livestock and poultry manure
PendingCN110734194AReduce economic costsEasy to operateWater treatment parameter controlWaste water treatment from animal husbandryChemistryBiotechnology
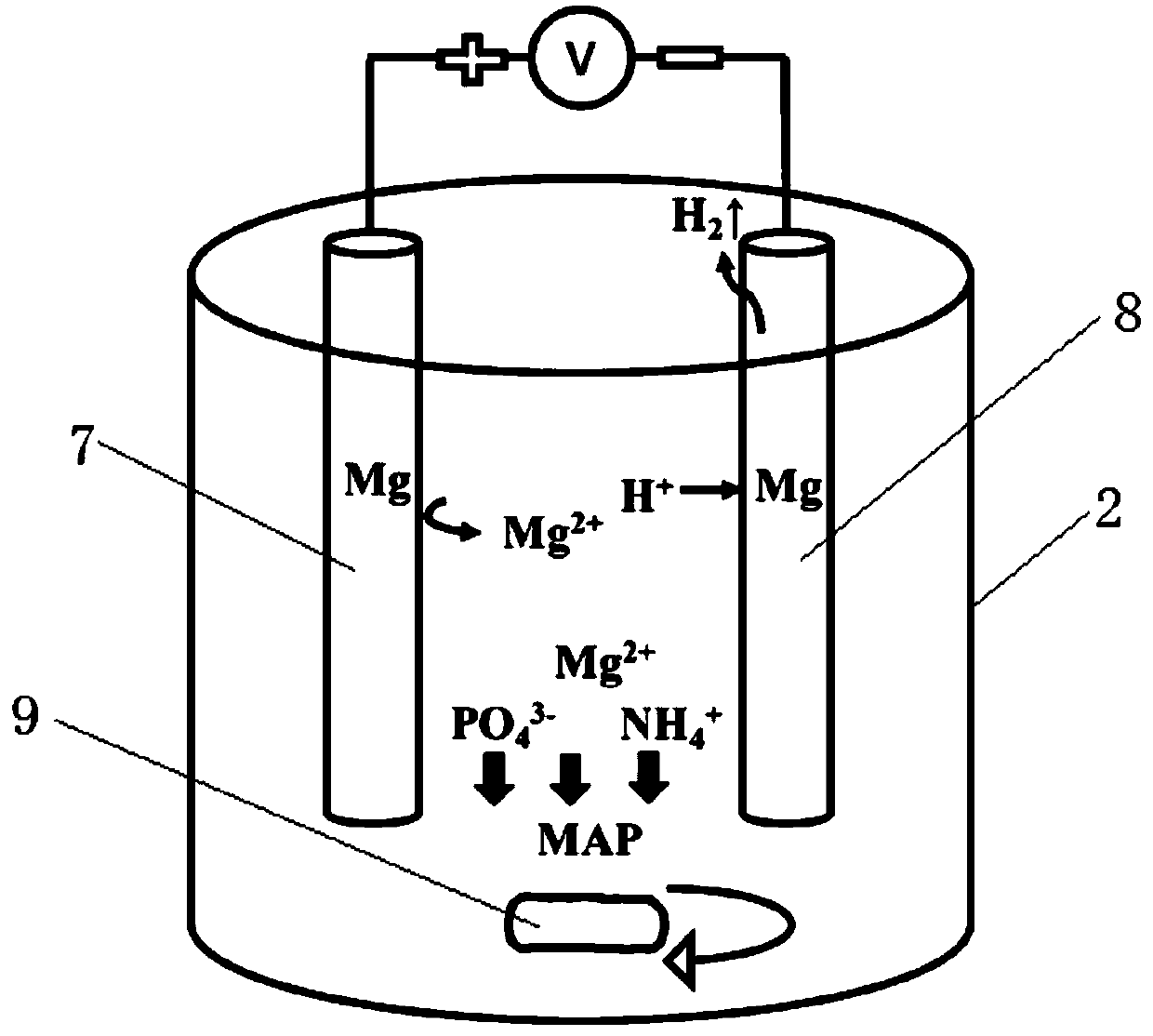
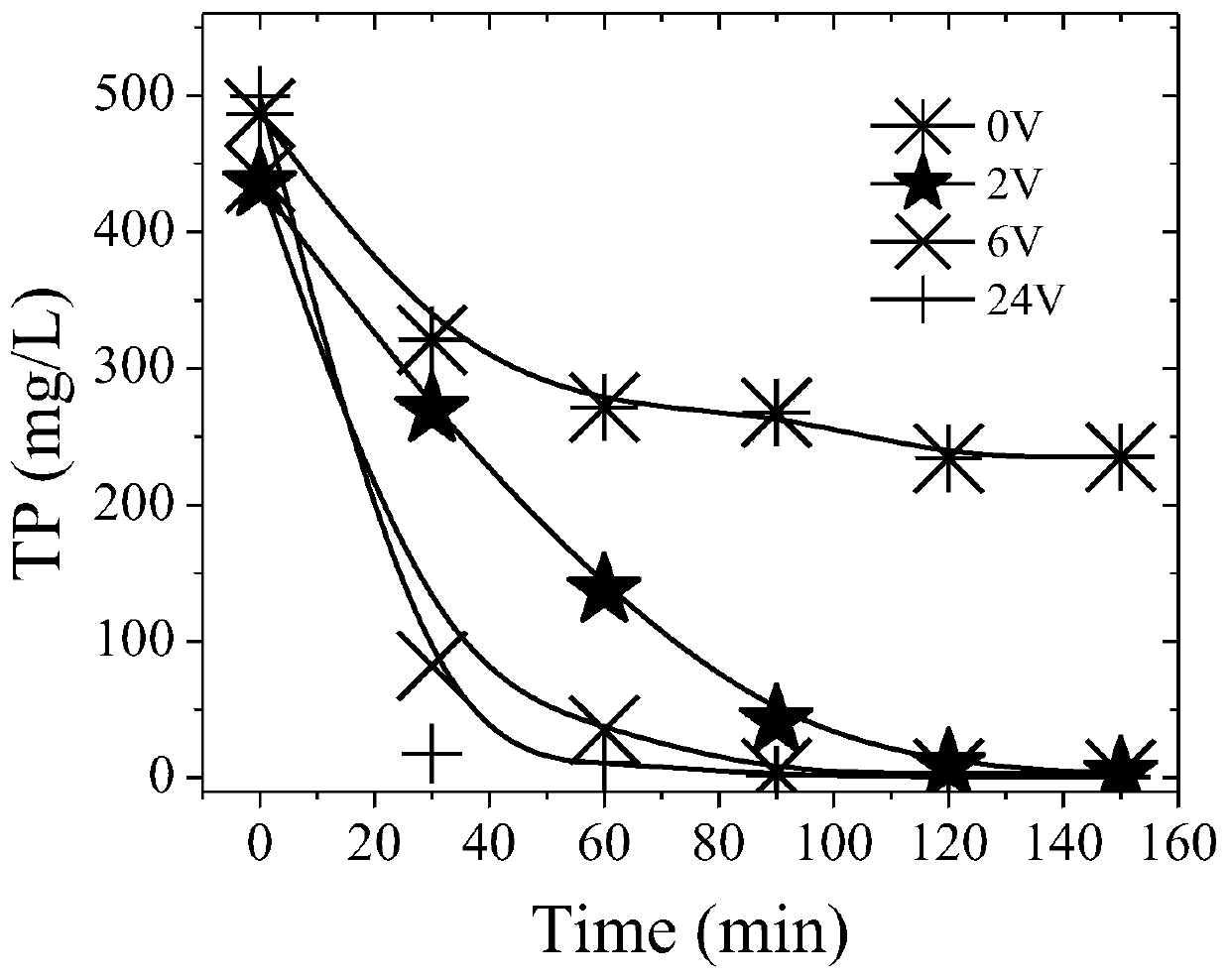
The invention discloses a method for recovering and purifying struvite from livestock and poultry manure, and the method comprises the following steps: putting the livestock and poultry manure into acontinuous flow reactor, and carrying out anaerobic fermentation to obtain manure digestive juice; introducing the manure digestive juice into an electrolytic reaction stirring tan, selecting magnesium as an anode of an electrode and selecting magnesium or metal of which the activity is lower than that of magnesium as a cathode, and electrolyzing under the stirring condition of a stirrer by usinga direct-current voltage; introducing the solution after the electrolytic reaction into a sedimentation tank for static sedimentation; introducing lower turbid liquid obtained after precipitation intoa heater; filtering the heated hot solution by using a filter film; and injecting clear liquid obtained after filtration of the filter film into a condensation recovery pool, and recovering the struvite solids. The method disclosed by the invention is low in cost, short in flow, simple to control, high in production efficiency, capable of separating the struvite from impurities, and suitable forindustrial popularization.
Owner:SHANDONG UNIV
Method for preparing large-particle lithium carbonate from salt lake lithium-rich brine
ActiveCN110217806ALess impurity ionsHigh yield and purityLithium carbonates/bicarbonatesLithium carbonateImpurity ions
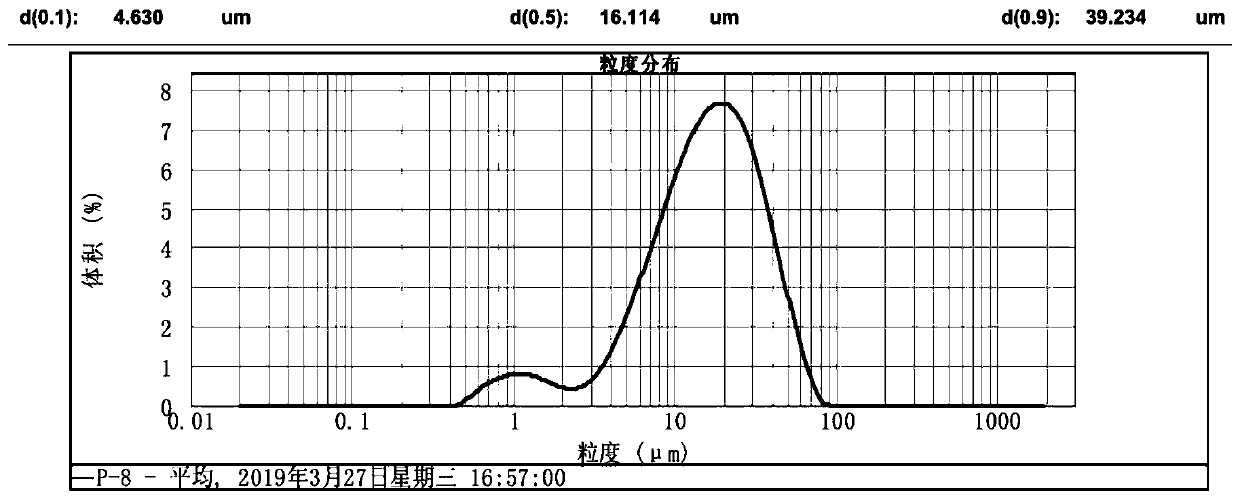
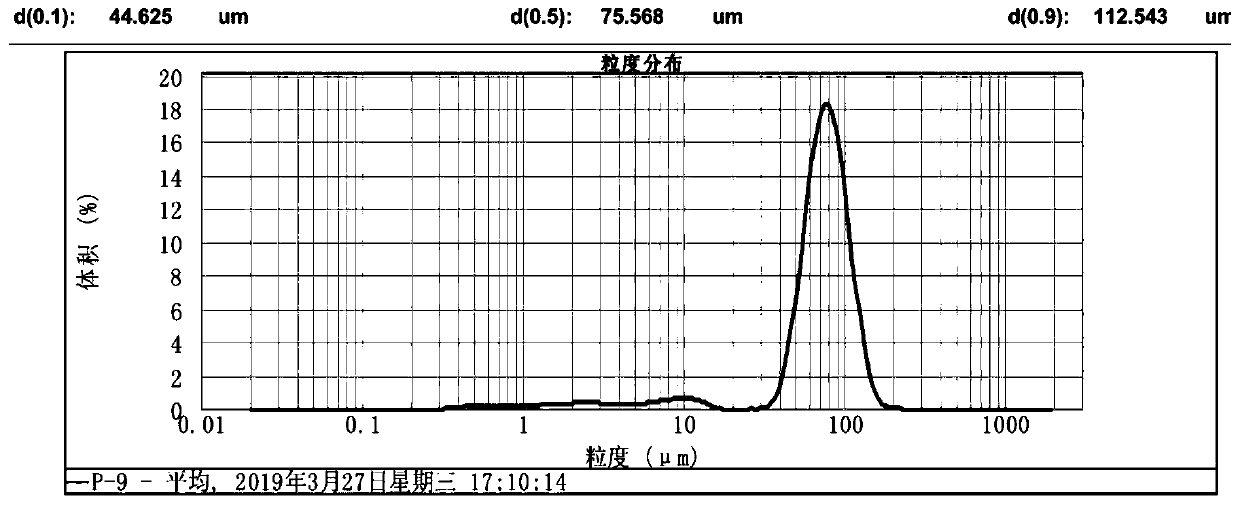
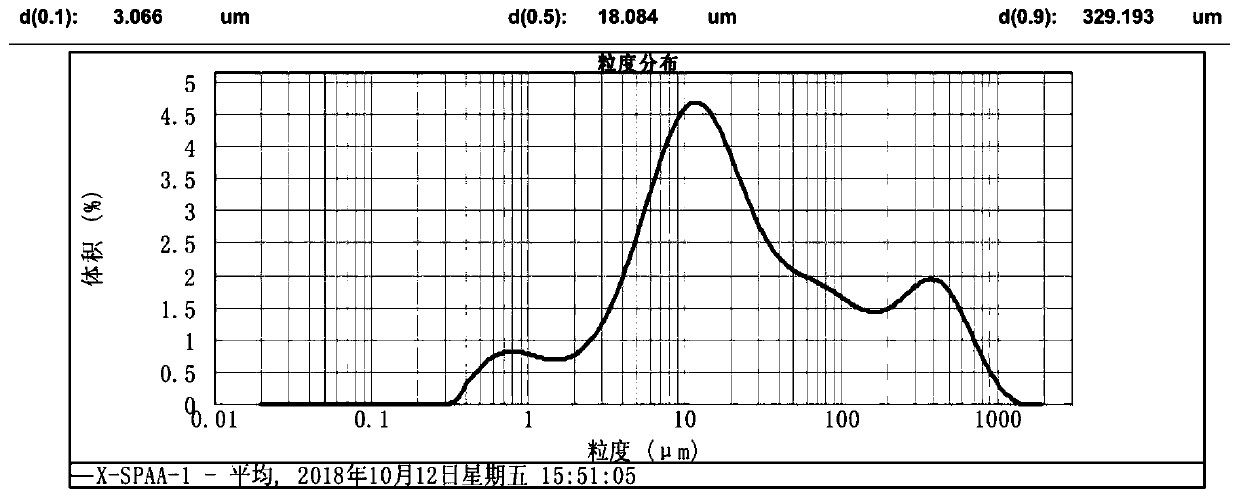
The invention discloses a method for preparing large-particle lithium carbonate from salt lake lithium-rich brine. The method comprises the following steps: step 1, heating the salt lake lithium-richbrine to 70-85 DEG C, after heating is completed, maintaining the temperature and adding a surfactant into the salt lake lithium-rich brine; step 2, maintaining the temperature in the step 1, adding acarbonate solution into the pre-treated salt lake lithium-rich brine, and performing a reaction for 1-3 h to obtain reaction product slurry; and step 3, allowing the reaction product slurry to standfor 10-20 h, performing solid-liquid separation, washing the obtained solid particles, and performing drying to obtain the large-particle lithium carbonate. According to the method provided by the invention, the certain surfactant is added into the reaction system, so that a particle size of the lithium carbonate is increased in the precipitation process, and the obtained lithium carbonate has a particle size d(0.9) of 300-600 [mu]m; and meanwhile impurity ions adsorbed at the surface of the lithium carbonate product are reduced, and purity and a yield of a primary product are improved, wherein the purity of the primary product is >=95%.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Aqueous metal anti-rust agent
The invention discloses an aqueous metal anti-rust agent containing cyclohexylamine citrate. The aqueous metal anti-rust agent has good anti-rust effects on iron, steel, iron alloys, aluminum, copper and the like, and thus can be utilized for rust protection of metal equipment and parts, wherein the metal equipment and parts are made of iron, steel, iron alloys, aluminum, copper and the like.
Owner:广州化学试剂厂
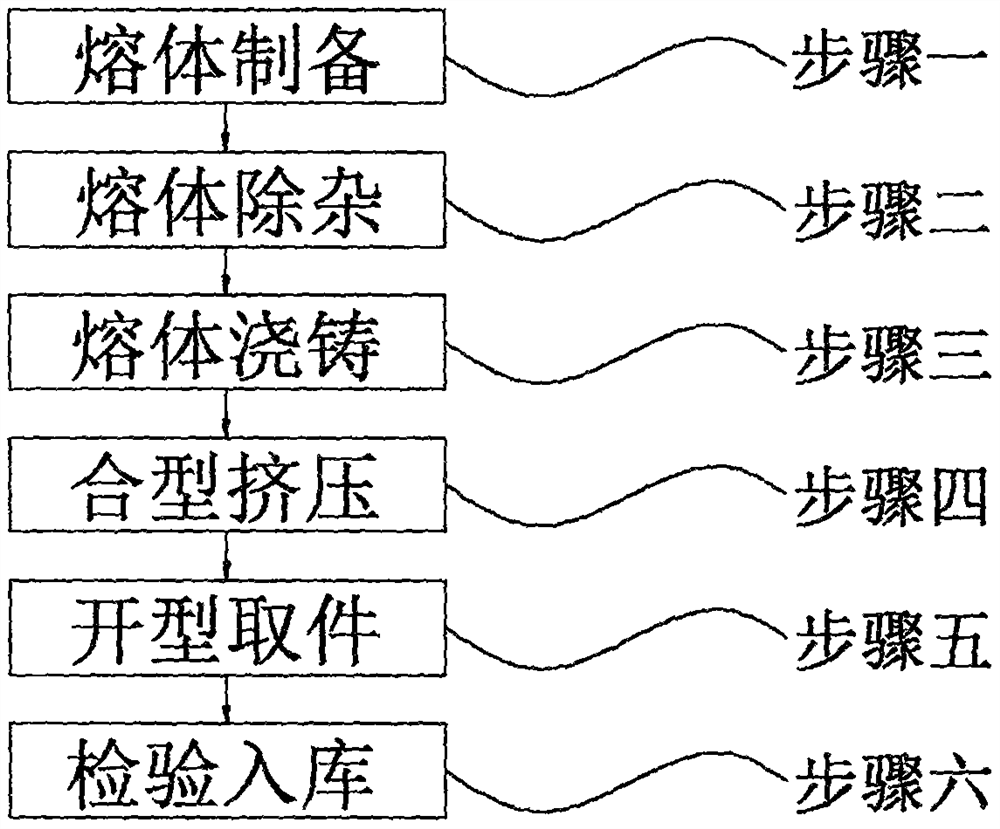
Low-cost extrusion casting process for aluminum-titanium-boron refiner
The invention discloses a low-cost extrusion casting process for an aluminum-titanium-boron refiner. The low-cost extrusion casting process comprises the following steps of step 1, preparing a melt; step 2, removing impurities from the melt; step 3, casting the melt; step 4, mold assembly and extrusion; step 5: opening mold and taking a part; and step 6, inspecting and warehousing. In the first step, titanium salt is potassium fluotitanate, boron salt is potassium fluoborate, and the temperature of molten aluminum is 800 DEG C. Compared with an existing manufacturing process of an aluminum-titanium-boron refiner, multiple impurity removal procedures are designed, impurity ions of alloy melt can be effectively reduced, the purity of the aluminum-titanium-boron refiner can be improved, and compared with an existing casting process, an aluminum-titanium-boron refiner casting is produced through the extrusion casting process, the alloy melt can be rapidly solidified under the high pressure, uniform distribution of active property points in the refiner is achieved, aggregation and clustering are avoided, the yield of the method is high, automatic production is convenient to achieve, and the labor cost is reduced.
Owner:LIAONING PROVINCIAL COLLEGE OF COMM
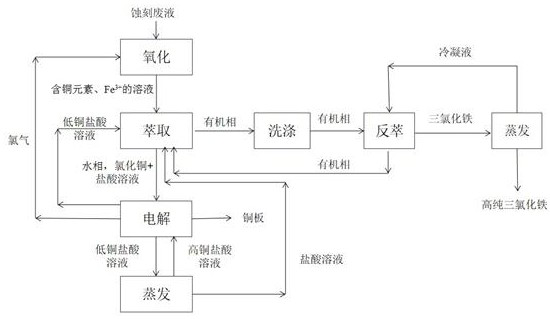
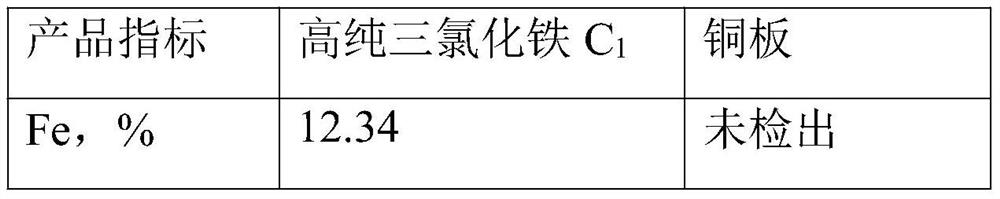
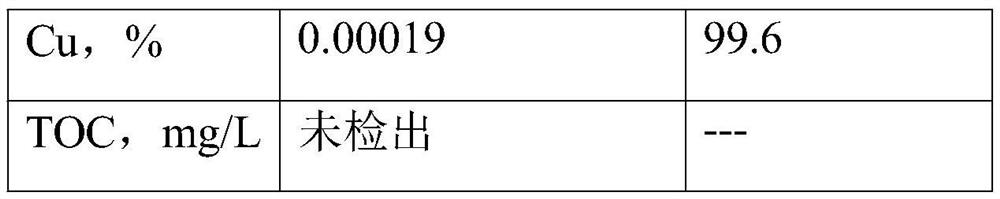
Method for recovering copper and iron from etching waste liquid
PendingCN113213547AEasy to separateHigh recovery ratePhotography auxillary processesIron compounds preparationElectrolytic agentEtching
The invention discloses a method for recovering copper and iron from etching waste liquid. The method comprises the following steps: oxidizing the etching waste liquid containing copper and iron elements into a ferric trichloride solution containing the copper element, adding industrial hydrochloric acid and butyl acetate for extraction, standing for layering, taking an organic phase, adding an iron-containing and acid-containing washing solution for washing the organic phase, and adding a stripping agent into the organic phase for stripping, to obtain ultralow-impurity ferric trichloride, and evaporating and concentrating to remove organic matters to obtain the high-purity ferric trichloride. The organic phase can be recycled, a water phase is extracted for multiple times until no ferric trichloride exists, a high-purity copper plate can be obtained through an electrolytic copper extraction process, and a low-copper electrolyte can be electrolyzed again through evaporation and concentration or can be used as hydrochloric acid required by extraction. The copper content of the high-purity ferric trichloride product produced by the process is lower than 5 ppm, and the high-purity ferric trichloride product can be applied to the fields of drinking water treatment, medical intermediates, analysis and testing, precise etching and the like; and the copper plate obtained by electrolysis has high purity and high value.
Owner:3R ENVIRONMENTAL TECH CO LTD
Prepn process of magnesia for silicon steel
The present invention relates to preparation process of magnesium compound, and is especially preparation process of magnesia for silicon steel. The present invention produces silicon steel level magnesia through a bittern-sodium carbonate process, which includes reaction of refined magnesium chloride solution and refined sodium carbonate solution at 65-85 deg.c for 5-15 min to produce basic magnesium carbonate, curing, filtering and calcining at 1100+ / -50 deg.c for 2.0+ / -0.5 hr. Thus produced silicon steel level magnesia has high purity, small average grain size, good coating performance, high adhesivity and other features, and the preparation process is simple and low in cost.
Owner:山西银海锋源新材料有限公司
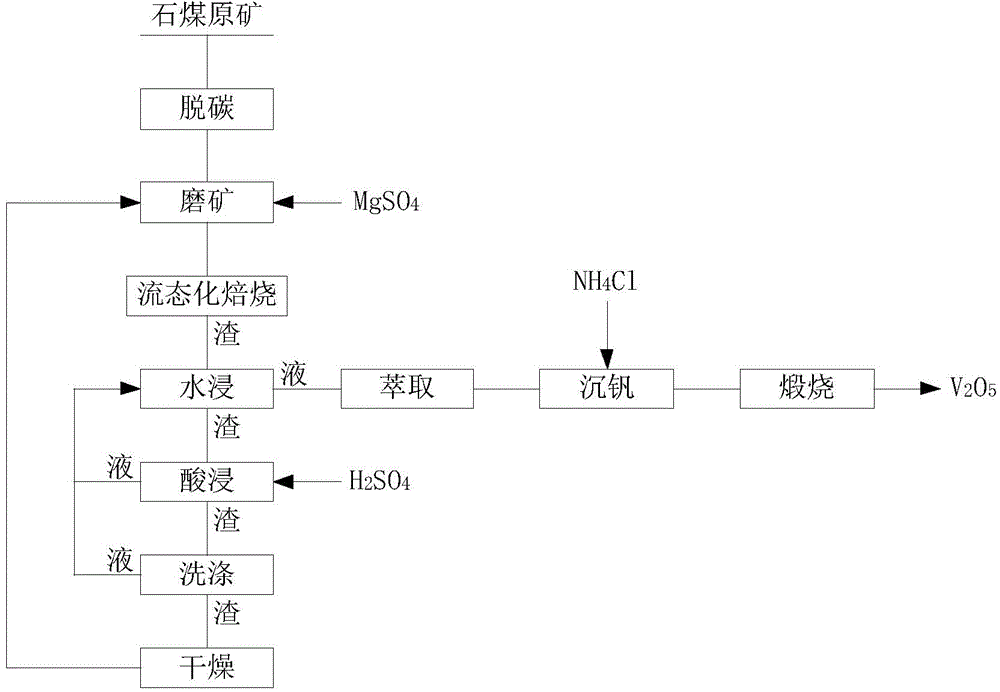
Method for recovering vanadium by virtue of magnesiation and roasting of stone coal
InactiveCN104911335AEmission reductionReduce water consumptionProcess efficiency improvementPregnant leach solutionRecovery method
The invention belongs to the technical field of mineral processing and particularly relates to a method for recovering vanadium by virtue of magnesiation and roasting of stone coal. The technical scheme is as follows: decarburizing stone coal ores, then adding MgSO4, performing fine grinding until the granularity is not greater than 100 meshes, performing fluosolid roasting, and performing water leaching on the roasted sample at a certain temperature, thereby obtaining vanadium-containing leachate; leaching the water leaching dregs with sulphuric acid, washing the leaching dregs with water, mixing acid leaching liquor with a washing solution so as to obtain a mixture used for leaching roasting dregs during water leaching, drying washed residues, mixing the dried washed residues with the decarburized stone coal ore, and performing ore grinding; and performing extraction and impurity removal on the obtained vanadium-containing leachate, then adding an NH4Cl solution for molybdenum precipitation, and incinerating the precipitate to obtain V2O5. The method is used for processing stone coal vanadium ore with vanadium content of no less than 0.80%; the recovery rate of vanadium in the obtained leachate is no less than 75%. According to the method, less harmful gases are discharged in a roasting process, the water consumption and the acid consumption are less, the production cost is low, solids and liquids can be recycled, and the application potential is great, so that the method is an economical and effective vanadium recovery method.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
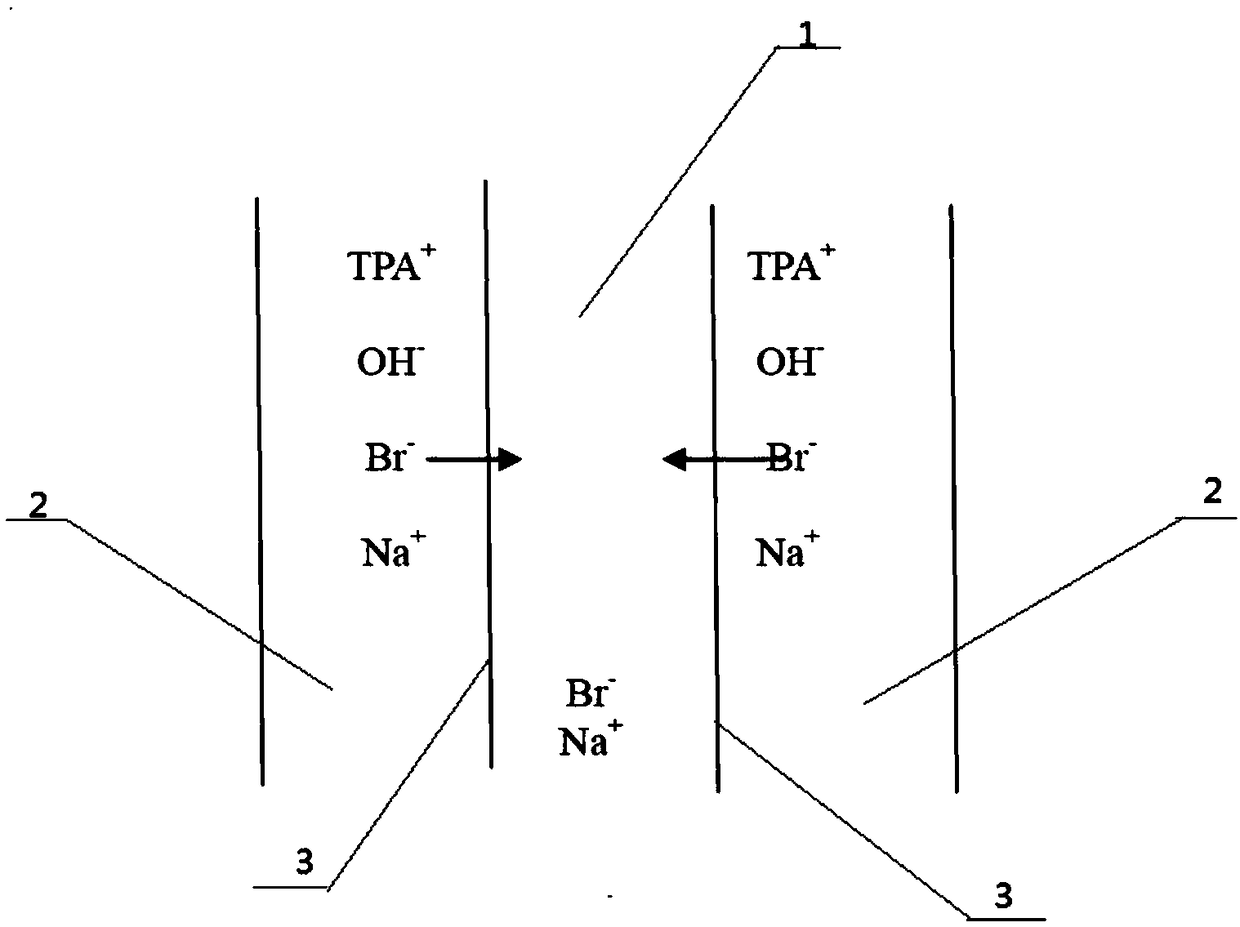
Method for purifying tetrapropylammonium hydroxide based on diffusion dialysis
InactiveCN109206322APurification effectLess impurity ionsAmino compound purification/separationPurification methodsImpurity ions
The invention provides a method for purifying tetrapropylammonium hydroxide based on diffusion dialysis. The method is characterized in that a diffusion dialysis device is used for purification; the diffusion dialysis device comprises an alkali chamber, a water chamber and an anion exchange membrane arranged between the alkali chamber and the water chamber; a tetrapropylammonium hydroxide solutionis added into the alkali chamber, and water is added into the water chamber, wherein the volumes of the tetrapropylammonium hydroxide solution and the water are identical; and static treatment in thediffusion dialysis device is performed for 80 to 110 h so as to complete purification. The purification method of the invention can effectively reduce impurity ions in tetrapropylammonium hydroxide,especially bromide ions; purification operation is simplified, and no manual intervention is needed in the process of purification, so the convenience of related operations is significantly improved;in addition, since the tetrapropylammonium hydroxide is purified by dynamic diffusion dialysis in the purification process, other chemicals are not involved, so no related waste materials are produced.
Owner:南京元亨化工科技有限公司
A kind of red fluorescent powder for near ultraviolet light excitation and preparation method thereof
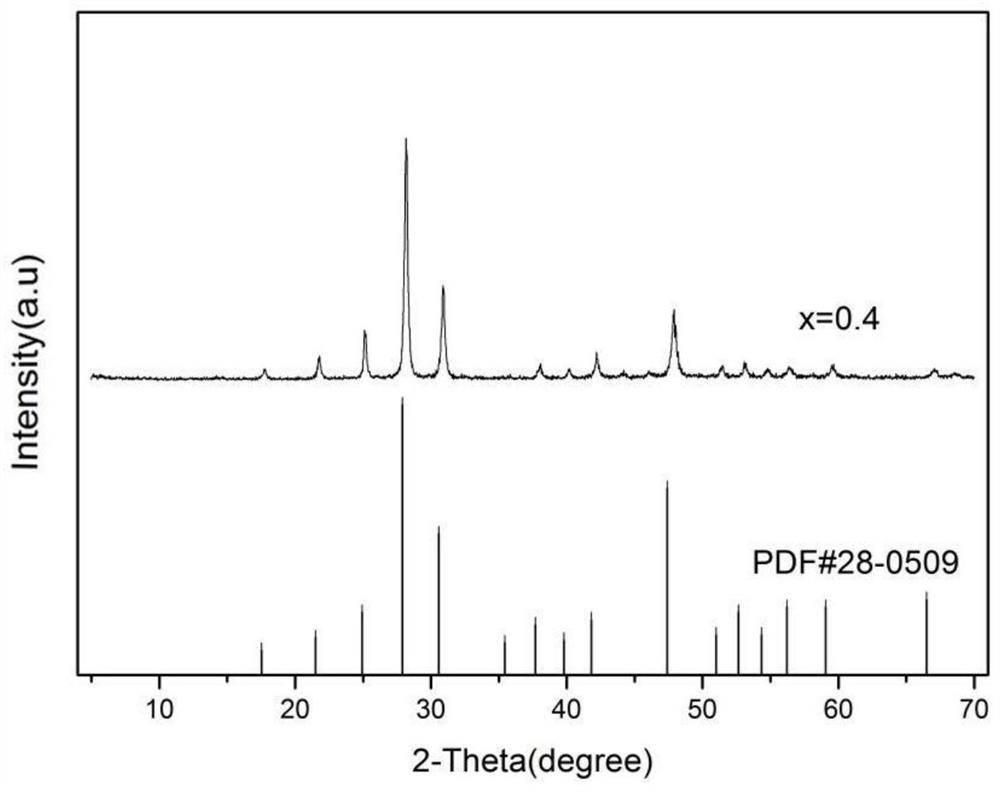
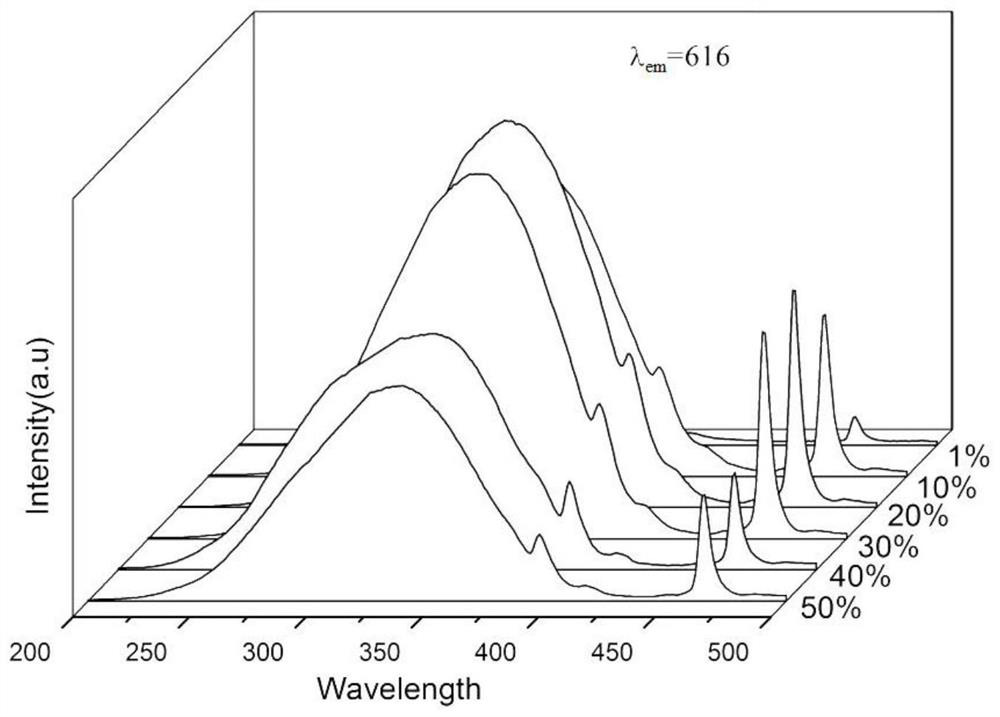
ActiveCN110157427BLuminous intensity can be controlledHigh purityLuminescent compositionsSemiconductor devicesHydration reactionLuminous intensity
Owner:SHAANXI UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com